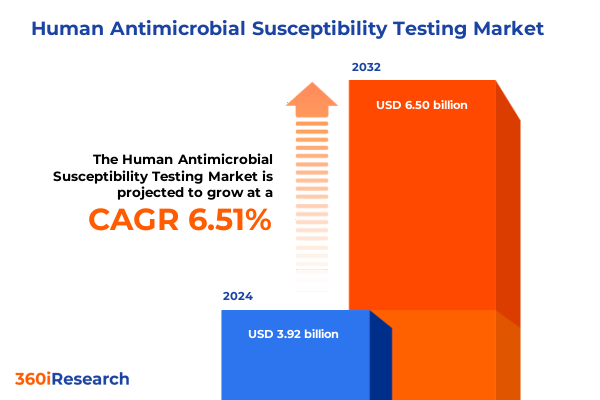
The Human Antimicrobial Susceptibility Testing Market size was estimated at USD 4.16 billion in 2025 and expected to reach USD 4.43 billion in 2026, at a CAGR of 6.55% to reach USD 6.50 billion by 2032.
Unveiling the Critical Importance and Emerging Dynamics of Human Antimicrobial Susceptibility Testing in Today's Healthcare Ecosystem
Antimicrobial susceptibility testing (AST) lies at the heart of contemporary infectious disease management, offering clinicians the critical data needed to tailor antibiotic therapies and combat the escalating threat of resistance. As pathogens evolve rapidly, traditional empiric treatments become increasingly ineffective, underscoring the urgency for accurate, timely susceptibility profiles. According to the World Health Organization, antimicrobial resistance threatens the effective prevention and treatment of an ever-increasing range of infections, amplifying morbidity and mortality worldwide.
The global burden of bacterial resistance is staggering, with recent industry data highlighting that antimicrobial-resistant infections contribute to nearly five million deaths each year, a figure that eclipses many other high-profile health crises. This executive summary provides a strategic overview of the human AST market, mapping the transformative trends, regulatory influences, segmentation nuances, regional dynamics, and competitive landscapes reshaping the industry today. Through these insights, decision-makers will be equipped to navigate challenges and harness opportunities in this critical healthcare domain.
Embracing a New Era of Diagnostic Innovation Where Automation, AI Integration, and Molecular Technologies Reshape Antimicrobial Susceptibility Testing
The antimicrobial susceptibility testing landscape is undergoing a paradigm shift driven by automation and advanced analytics. Laboratories are rapidly integrating automated AST platforms that reduce hands-on time and enhance reproducibility, while artificial intelligence algorithms now enable real-time interpretation of complex data sets. Industry analyses predict that these AI-powered systems will become central to laboratory operations, optimizing workflows and uncovering resistance patterns with unprecedented speed and accuracy. Moreover, the integration of informatics solutions by leading manufacturers underscores the move toward intelligent, connected diagnostics that support antimicrobial stewardship programs and institutional quality metrics.
Concurrently, molecular methods are gaining traction as complements to phenotypic tests, allowing rapid detection of resistance genes and enabling clinicians to make more informed treatment decisions. Point-of-care AST devices, once niche products in resource-limited settings, are now evolving into robust tools for bedside diagnostics, further democratizing access to susceptibility data. Cloud-based platforms and digital connectivity are also emerging as essential components of modern AST, facilitating data sharing across geographies and supporting global surveillance efforts.
Assessing the Far-Reaching Consequences of 2025 United States Tariff Policies on Antimicrobial Susceptibility Testing Supply Chains and Cost Structures
In early 2025, U.S. tariff policies introduced a universal 10 percent levy on most imported goods, with country-specific adjustments following days later. China now faces cumulative duties of up to 145 percent on lab-related imports, while Canada and Mexico are subject to 25 percent tariffs on non-USMCA goods and 10 percent on energy and potash. Laboratories dependent on imported instruments, reagents, and consumables have been forced to reassess supplier portfolios, prioritizing domestic sourcing and vertical integration to contain costs and safeguard operations. This rapid escalation in trade barriers has exposed vulnerabilities across AST supply chains, prompting industry stakeholders to explore localization strategies and strategic stockpiling to mitigate future disruptions.
Beyond levies on basic lab equipment, Section 301 tariffs targeting specific medical devices and consumables have imposed additional pressures on diagnostic budgets. A comprehensive Baker McKenzie analysis highlights that diagnostic reagents classified under medical devices now face duties that significantly increase the cost per test, pressuring both high-volume reference labs and smaller clinical facilities. Furthermore, insights from GlobalData underscore the likelihood that sustained tariff pressures will accelerate supply chain diversification, incentivizing manufacturers to invest in regional manufacturing hubs and resilient sourcing frameworks.
In-Depth Analysis of Market Segmentation Techniques Revealing How Product, Technology, Test Type, Application, End User, and Pathogen Criteria Drive Industry Growth
The human AST market can be dissected through multiple segmentation lenses, each revealing unique drivers and adoption patterns. Product segmentation distinguishes instruments-ranging from benchtop analyzers to fully walk-away systems-from reagents and consumables such as discs, kits, media, and strips, while software platforms underpin laboratory information management and decision support. Technology segmentation further categorizes solutions into automated systems, disk diffusion, gradient strip, microdilution, and molecular methods, highlighting how automation platforms like MicroScan, Phoenix, and Vitek Systems are reshaping throughput and result consistency.
Test type segmentation contrasts automated testing with manual methodologies, the latter encompassing agar dilution, broth microdilution, disk diffusion, and gradient strips, each retaining a role in specific clinical and reference lab workflows. Application segmentation underscores that clinical diagnostics remains the primary driver of AST utilization, with pharmaceutical drug development and academic research fueling specialized technology adoption. End-user segmentation reveals that clinics and diagnostic laboratories lead in volume testing, while hospitals and research institutes drive demand for high-complexity assays. Pathogen segmentation emphasizes a diversification of focus from bacteria-Gram-negative Enterobacteriaceae and non-Enterobacteriaceae, Gram-positive species-to fungi and mycobacteria, reflecting the growing importance of fungal susceptibility testing in light of rising invasive fungal diseases.
This comprehensive research report categorizes the Human Antimicrobial Susceptibility Testing market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Product
- Technology
- Test Type
- Pathogen
- Application
- End User
Critical Regional Perspectives Highlighting Distinct Drivers, Challenges, and Opportunities Across the Americas, EMEA, and Asia-Pacific Antimicrobial Markets
In the Americas, the United States unquestionably leads the AST landscape, buoyed by a robust healthcare infrastructure, substantial R&D investment, and stringent regulatory frameworks that prioritize antimicrobial stewardship. Government initiatives such as the National Action Plan for Combating Antibiotic-Resistant Bacteria have further solidified this leadership, driving the early adoption of innovative AST solutions and enabling rapid clinical integration of automated and molecular platforms.
Within Europe, the Middle East, and Africa, policy-driven growth underpins market expansion. The European Commission’s renewed One Health Action Plan against AMR harmonizes surveillance, infection prevention, and R&D incentives across member states, fostering a collaborative ecosystem between laboratories, policymakers, and industry stakeholders. This regulatory cohesion, coupled with funding streams through EU4Health and Horizon Europe, has catalyzed the adoption of advanced AST technologies in both mature and emerging markets across the region.
Asia-Pacific markets are characterized by rapid healthcare modernization and expanding laboratory networks. Governments in countries such as China, India, Australia, and Japan are investing heavily in AMR surveillance laboratories and digital reporting platforms, aligning with WHO’s GLASS framework to enhance data quality and coverage. As of late 2023, 135 countries and territories participate in GLASS, reflecting a collective push toward standardized AMR data collection and fostering demand for scalable AST solutions that support national and regional ambitions.
This comprehensive research report examines key regions that drive the evolution of the Human Antimicrobial Susceptibility Testing market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Strategic Competitive Insights Examining How Leading Diagnostic and Life Science Companies Are Steering Technological Advancements in Antimicrobial Susceptibility Testing
Leading diagnostic manufacturers are channeling innovation into streamlined workflows and integrated informatics. Becton Dickinson’s recent FDA 510(k) clearance for its BD Phoenix™ M50 and BDXpert™ informatics solution exemplifies the sector’s pivot toward automating end-to-end microbiology processes, from rapid identification to susceptibility interpretation, thereby addressing staffing shortages and accelerating result turnaround times. BioMérieux has similarly fortified its product portfolio with FDA clearance of the Vitek Compact Pro, a compact ID/AST system designed to deliver high-quality results in small to medium clinical laboratories, signaling a trend toward modular, space-efficient instrumentation.
Innovators like Accelerate Diagnostics are pushing the boundaries of rapid testing, with its WAVE™ system submission poised to deliver same-shift AST directly from positive blood cultures, potentially reducing the window to targeted therapy to under five hours. Beyond traditional device manufacturers, life science conglomerates such as Abbott Laboratories are responding to geopolitical trade pressures by investing over $500 million in U.S. manufacturing capacity for diagnostics and medical devices, underscoring the strategic prioritization of domestic supply chain resilience.
This comprehensive research report delivers an in-depth overview of the principal market players in the Human Antimicrobial Susceptibility Testing market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Accelerate Diagnostics Inc.
- Becton, Dickinson and Company
- bioMérieux SA
- Bruker Corporation
- Danaher Corporation
- F. Hoffmann-La Roche AG
- HiMedia Laboratories Pvt. Ltd.
- Liofilchem S.r.l.
- Luminex Corporation
- QIAGEN N.V.
- Siemens Healthineers AG
- Thermo Fisher Scientific Inc.
Actionable Strategic Recommendations Guiding Industry Leaders to Enhance AST Laboratory Efficiency, Bolster Supply Chain Resilience, and Forge Collaborative Partnerships
Industry leaders should prioritize the deployment of automated and AI-driven AST platforms to optimize laboratory throughput, reduce manual intervention, and improve data accuracy. By integrating informatics solutions and advanced analytics, organizations can enhance diagnostic stewardship and support clinician decision-making with actionable, real-time susceptibility data.
To mitigate ongoing trade and supply chain risks, stakeholders must accelerate diversification of sourcing and invest in regional manufacturing hubs. Strategic partnerships with domestic suppliers, coupled with inventory optimization and demand forecasting, will be critical to ensuring uninterrupted access to key reagents, consumables, and instrumentation.
Collaborative frameworks between diagnostic developers, public health agencies, and clinical laboratories can drive standardized data sharing and surveillance efforts. Embracing cloud-based platforms and adhering to international reporting protocols will strengthen AMR monitoring and foster cross-sectoral insights to inform policy and R&D prioritization.
Investing in workforce training and certification programs is essential to maximize the capabilities of next-generation AST systems. Upskilling laboratory personnel in molecular methods, data analytics, and IT management will enhance operational resilience and ensure the efficient utilization of advanced testing solutions.
Comprehensive Research Methodology Detailing Our Rigorous Primary and Secondary Data Collection, Expert Interviews, and Analytical Frameworks Validating AST Market Insights
This market analysis is grounded in a rigorous research framework combining primary and secondary methodologies. Primary insights were derived from in-depth interviews with laboratory directors, procurement specialists, regulatory experts, and R&D leaders across clinical, pharmaceutical, and academic settings. Secondary research encompassed peer-reviewed journals, regulatory filings, industry white papers, and authoritative agency publications.
Quantitative data were validated through triangulation across multiple sources, ensuring the reliability of emerging trend identification. Analytical models incorporated segmentation analyses, regional drivers, and competitive benchmarking, while scenario planning exercises evaluated potential policy and technological shifts. Expert panels reviewed findings to align perspectives and refine strategic recommendations, providing a robust basis for market conclusions and actionable guidance.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Human Antimicrobial Susceptibility Testing market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Human Antimicrobial Susceptibility Testing Market, by Product
- Human Antimicrobial Susceptibility Testing Market, by Technology
- Human Antimicrobial Susceptibility Testing Market, by Test Type
- Human Antimicrobial Susceptibility Testing Market, by Pathogen
- Human Antimicrobial Susceptibility Testing Market, by Application
- Human Antimicrobial Susceptibility Testing Market, by End User
- Human Antimicrobial Susceptibility Testing Market, by Region
- Human Antimicrobial Susceptibility Testing Market, by Group
- Human Antimicrobial Susceptibility Testing Market, by Country
- United States Human Antimicrobial Susceptibility Testing Market
- China Human Antimicrobial Susceptibility Testing Market
- Competitive Landscape
- List of Figures [Total: 18]
- List of Tables [Total: 1908 ]
Drawing Strategic Conclusions on Future Opportunities and Navigating Challenges in the Antimicrobial Susceptibility Testing Landscape
The human antimicrobial susceptibility testing market stands at a pivotal juncture, with technological advancements and shifting regulatory landscapes converging to redefine laboratory practices and patient outcomes. Automation, AI integration, and molecular diagnostics are driving a swift transition from traditional phenotypic methods to high-throughput, precision-oriented workflows. These innovations, coupled with policy initiatives and regional investments, are expanding access to vital AST services and reinforcing global efforts to contain antimicrobial resistance.
Looking ahead, stakeholders who embrace data connectivity, supply chain diversification, and collaborative partnerships will be best positioned to capitalize on emerging opportunities. As the market evolves, aligning strategic imperatives with public health objectives and technological capabilities will be essential to sustaining momentum and delivering impactful, patient-centered solutions.
Connect with Ketan Rohom to Access the Complete Human Antimicrobial Susceptibility Testing Market Research Report for Unparalleled Strategic Insights
Ready to deepen your understanding of the human antimicrobial susceptibility testing market and gain a competitive edge? Reach out to Ketan Rohom, Associate Director, Sales & Marketing at 360iResearch, to explore the full breadth of insights this comprehensive report offers. Discover tailored analyses, detailed segment breakdowns, and strategic forecasts designed to empower your organization.
Engage today to secure your copy of the report and receive expert guidance on leveraging key findings to drive innovation, optimize operations, and capitalize on emerging market opportunities.

- How big is the Human Antimicrobial Susceptibility Testing Market?
- What is the Human Antimicrobial Susceptibility Testing Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




