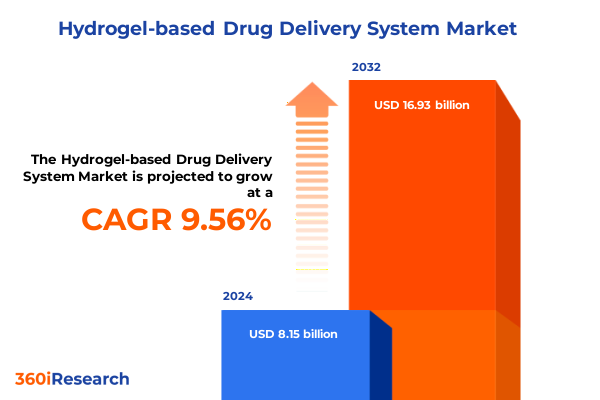
The Hydrogel-based Drug Delivery System Market size was estimated at USD 8.89 billion in 2025 and expected to reach USD 9.70 billion in 2026, at a CAGR of 9.63% to reach USD 16.93 billion by 2032.
Revolutionizing Therapeutics with Hydrogel Matrices That Enhance Targeted Delivery and Patient Outcomes in Modern Healthcare Landscapes
Hydrogel matrices represent a groundbreaking frontier in pharmaceutical science, offering unparalleled opportunities to refine drug delivery modalities. By harnessing the unique physicochemical properties of three-dimensional polymer networks, hydrogel-based systems achieve controlled release kinetics, site-specific targeting, and enhanced biocompatibility. These features collectively address longstanding challenges in therapeutic administration, such as minimizing systemic side effects and improving patient adherence. In this evolving landscape, clinicians and formulators alike are gravitating toward hydrogel platforms as enablers of precision medicine applications.
As the healthcare industry shifts toward personalized treatment paradigms, hydrogels stand out for their ability to encapsulate a diverse array of bioactive molecules-from small-molecule chemotherapeutics to high-molecular-weight biologics-without compromising molecular stability. Furthermore, advances in polymer chemistry permit the fine-tuning of mechanical strength, degradation rates, and stimuli responsiveness, elevating hydrogels above traditional delivery vehicles. This executive summary sets the stage for an in-depth exploration of the hydrogel-based drug delivery system market by illuminating prevailing trends, regulatory influences, and segmentation dynamics. Through this lens, organizations can navigate complex opportunities and chart strategic initiatives that align with the state-of-the-art in hydrogel innovations.
Emerging Material Innovations and Regulatory Advances Reshaping Hydrogel-Based Drug Delivery toward Precision Therapeutics and Sustainable Manufacturing
Recent years have witnessed a confluence of technological breakthroughs that are redefining hydrogel-based drug delivery as a cornerstone of advanced therapy design. Innovations in polymer synthesis have yielded stimuli-responsive hydrogels that undergo conformational changes in response to pH, temperature, or specific enzymatic activity, thereby enabling on-demand release profiles. Concurrently, the integration of nanotechnology with hydrogel scaffolds has opened avenues for multifunctional platforms capable of simultaneous imaging and therapy, laying the groundwork for theranostic applications.
On the regulatory front, expedited pathways and adaptive approval mechanisms for advanced drug delivery systems have reduced time-to-market while maintaining stringent safety standards. These frameworks have incentivized investment in hydrogel technologies, driving cross-sector collaborations among polymer chemists, biomedical engineers, and healthcare institutions. Moreover, sustainability considerations are prompting the development of green manufacturing processes for hydrogel monomers, addressing environmental impact without sacrificing material performance. Collectively, these transformative shifts mark a pivotal era in which hydrogel delivery platforms are poised to deliver unprecedented therapeutic precision and operational efficiency.
Evaluating the Far-Reaching Economic and Operational Consequences of 2025 US Tariff Policies on Hydrogel Supply Chains and Industry Competitiveness
The imposition of new US tariffs in 2025 on key polymer precursors and specialized additives has introduced significant cost dynamics into the hydrogel supply chain. These levies have elevated raw material prices, compelling manufacturers to reassess sourcing strategies and explore alternative suppliers both domestically and internationally. In response, some entities have initiated vertical integration of precursor production, reducing dependency on imported monomers and fortifying supply resilience.
Beyond cost pressures, tariff-induced disruptions have driven downstream effects on formulation development timelines and pricing strategies. Contract research organizations are negotiating cost-sharing arrangements, while strategic partnerships with local chemical engineers aim to optimize production yields. At the same time, biopharmaceutical companies are leveraging tariff exemptions and duty drawback programs to mitigate financial impact, ensuring continuity in clinical trials and product rollouts. From an operational standpoint, these policy shifts underscore the imperative for agile supply chain models, strategic inventory planning, and robust risk-management frameworks that can absorb external trade fluctuations without compromising innovation trajectories.
Unveiling Critical Insights into Application Scope Product Types Therapeutic Areas and End User Dynamics Driving Market Differentiation in Hydrogel Delivery Systems
Delineating the hydrogel delivery system landscape through an application-focused lens reveals differentiated growth drivers and clinical imperatives. In ophthalmic delivery, both eye drop formulations and ocular inserts capitalize on the inherent mucoadhesive properties of hydrogels to enhance corneal residence time, thereby improving therapeutic efficacy for conditions ranging from glaucoma to postoperative inflammation. Meanwhile, tissue engineering applications leverage hydrogel scaffolds for bone regeneration, cartilage repair, and skin substitute constructs, each demanding tailored mechanical and degradative characteristics to mimic native extracellular matrices effectively.
Transdermal delivery formulations utilize gels and patches to circumvent first-pass metabolism, delivering small molecules and macromolecules across the dermal barrier with precision. The wound healing segment benefits from hydrogel dressings designed for acute lacerations, burn care, and chronic venous ulcers by maintaining optimal moisture balance and facilitating controlled drug release. When product-type considerations are introduced, hybrid polymers that combine synthetic and natural matrix elements emerge as versatile platforms with tunable performance, while purely natural polymer systems appeal to biocompatibility mandates and fully synthetic analogs promise scalable manufacturing.
Therapeutic area segmentation further clarifies market contours, as cardiovascular therapies confront localized delivery challenges, diabetes management explores insulin-responsive matrices, oncology trials test intratumoral hydrogel depots, and orthopedic repair procedures integrate hydrogels for localized growth factor administration. Finally, end-user perspectives ranging from outpatient clinics and homecare settings to hospital wards and research institutes highlight variable adoption rates informed by infrastructure readiness and clinical training requirements.
This comprehensive research report categorizes the Hydrogel-based Drug Delivery System market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Product Type
- Therapeutic Area
- Application
- End User
Analyzing Distinctive Growth Patterns and Adoption Drivers across the Americas Europe Middle East Africa and Asia Pacific for Hydrogel Drug Delivery Solutions
Regional analysis reveals distinct patterns of hydrogel delivery system adoption and innovation across the Americas, Europe Middle East Africa, and Asia Pacific. In the Americas, robust clinical trial infrastructures and established regulatory frameworks have fueled rapid integration of novel hydrogel formulations into mainstream therapeutic protocols. North American biopharma companies, supported by academic research centers and venture capital networks, spearhead platform diversification and novel indication development.
Within the Europe Middle East Africa landscape, harmonized regulations in the European Union streamline cross-border market entry for hydrogel-based products, fostering collaboration between multinational pharmaceutical firms and regional material science pioneers. Emerging markets in the Middle East are investing in healthcare infrastructure to support advanced wound care and ophthalmology applications, while select African countries prioritize local manufacturing partnerships to enhance accessibility and drive down supply chain costs.
Asia Pacific stands out for aggressive expansion, with China and India ramping up domestic production of biocompatible polymers, and Japan focusing on precision delivery solutions for geriatric populations. Collaborative efforts among regulators, academic institutions, and private sector players in the region have accelerated innovation cycles, making Asia Pacific a critical growth engine with competitive pricing and rapid adoption curves.
This comprehensive research report examines key regions that drive the evolution of the Hydrogel-based Drug Delivery System market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Profiling Leading Innovators and Strategic Collaborators in the Hydrogel Drug Delivery Space Highlighting Competitive Positioning and Technological Leadership
The competitive arena of hydrogel-based drug delivery systems is anchored by companies that blend materials science expertise with pharmaceutical development acumen. Trendsetting organizations have cultivated proprietary polymer libraries and hold extensive patent portfolios that secure their lead in performance metrics such as release kinetics, mechanical integrity, and biodegradation profiles. Strategic alliances between specialty polymer manufacturers and global biopharma corporations have become instrumental in co-developing application-specific hydrogel platforms optimized for targeted indications.
Mid-tier innovators are carving out niches through focused investments in personalized medicine and patient-centric design, often collaborating with digital health startups to embed sensors or wireless monitoring features within hydrogel matrices. This convergence of material engineering and digital therapeutics offers real-time feedback on drug release and tissue response, enhancing treatment personalization. Concurrently, contract manufacturing organizations with hydrogel processing capabilities are scaling flexible production lines to support clinical and commercial volumes, positioning themselves as critical partners for companies seeking rapid market entry and supply diversity.
Across the board, intellectual property strategies revolve around platform extensibility, enabling core hydrogel technologies to be repurposed across multiple therapeutic areas and delivery routes. These competitive maneuvers underscore an industry trajectory that balances high-value differentiation with collaborative ecosystems to bring next-generation delivery systems to clinical reality.
This comprehensive research report delivers an in-depth overview of the principal market players in the Hydrogel-based Drug Delivery System market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- 3M Company
- Ashland Global Holdings Inc.
- BASF SE
- Bausch + Lomb Corporation
- Blairex Laboratories, Inc.
- Dow Inc.
- DSM N.V.
- DuPont de Nemours, Inc.
- Endo International plc
- Evonik Industries AG
- Ferring B.V.
- Galderma S.A.
- Johnson & Johnson
- Lonza Group AG
- Medtronic plc
- Merck KGaA
- Ocular Therapeutix, Inc.
- The Lubrizol Corporation
- Tolmar Pharmaceuticals, Inc.
Strategic Imperatives and Best Practice Pathways for Industry Leaders to Capitalize on Hydrogel Delivery System Innovations and Market Opportunities
Industry leaders must prioritize multi-stakeholder collaboration to accelerate the translation of hydrogel innovations from bench to bedside. Establishing strategic partnerships with academic institutions and clinical centers will diversify the innovation pipeline while ensuring alignment with real-world therapeutic requirements. In parallel, organizations should invest in sustainable sourcing of polymer precursors and adopt circular economy principles to mitigate environmental impact and enhance supply chain resilience.
Early engagement with regulatory bodies is essential to navigate evolving approval pathways for advanced drug delivery systems, allowing for adaptive trial designs and accelerated labeling authorizations. Additionally, developing modular manufacturing processes capable of handling both small-batch clinical supplies and scaled commercial volumes will future-proof operations against market fluctuations. Embedding digital monitoring technologies within hydrogel platforms can deliver actionable patient data, fostering a feedback-driven product development cycle that hones performance in real time.
Finally, a structured portfolio management approach that balances core platform enhancements with targeted indication expansions will optimize resource allocation. By combining robust R&D investments with strategic licensing and co-development deals, organizations can capture emerging market opportunities while safeguarding against tariff-driven cost volatility.
Detailing the Comprehensive Research Framework and Analytical Approaches Underpinning Data Integrity for the Hydrogel Based Drug Delivery System Study
This study was underpinned by a rigorous research design that integrates both qualitative and quantitative methodologies to ensure comprehensive coverage of hydrogel-based drug delivery systems. Primary data collection involved structured interviews with key opinion leaders, including polymer chemists, formulation scientists, regulatory experts, and clinical investigators, to capture nuanced insights into material performance and adoption barriers. These perspectives were systematically triangulated with secondary sources such as peer-reviewed journals, patents databases, and public regulatory filings to verify emerging trends and proprietary breakthroughs.
Quantitative data was sourced from validated industry databases, manufacturing records, and clinical trial registries to map out pipeline dynamics and supply chain structures. Statistical analyses, including correlation studies and scenario modeling, were employed to identify growth drivers, cost drivers, and risk factors. The research framework also incorporated a thorough assessment of tariff schedules, trade policy shifts, and regional regulatory landscapes to contextualize economic implications.
To maintain data integrity, all inputs underwent cross-validation through expert panel reviews and iterative feedback loops with internal analysts. The methodological approach ensures that conclusions presented in this report are grounded in robust evidence and offer actionable intelligence for strategic decision-making.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Hydrogel-based Drug Delivery System market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Hydrogel-based Drug Delivery System Market, by Product Type
- Hydrogel-based Drug Delivery System Market, by Therapeutic Area
- Hydrogel-based Drug Delivery System Market, by Application
- Hydrogel-based Drug Delivery System Market, by End User
- Hydrogel-based Drug Delivery System Market, by Region
- Hydrogel-based Drug Delivery System Market, by Group
- Hydrogel-based Drug Delivery System Market, by Country
- United States Hydrogel-based Drug Delivery System Market
- China Hydrogel-based Drug Delivery System Market
- Competitive Landscape
- List of Figures [Total: 16]
- List of Tables [Total: 1431 ]
Synthesizing Core Findings and Strategic Reflections to Illuminate Future Trajectories and Growth Catalysts within the Hydrogel Delivery System Sector
Through this analysis, it is evident that hydrogel-based drug delivery systems stand at the intersection of material science innovation and clinical necessity. Advances in polymer engineering, coupled with supportive regulatory pathways, have converged to deliver platforms capable of precise, controlled release across a spectrum of therapeutic areas. Simultaneously, evolving tariff landscapes have underscored the need for resilient supply chain strategies and agile operational models.
Segmentation insights reveal how application-specific requirements, product-type distinctions, therapeutic demands, and end-user contexts drive divergence in R&D and commercialization approaches. Regional analyses highlight the Americas, Europe Middle East Africa, and Asia Pacific as critical arenas for market expansion, each with unique adoption enablers and infrastructural considerations. Competitive evaluations underscore the value of intellectual property, collaborative networks, and manufacturing flexibility as differentiators in a rapidly maturing market.
Looking forward, the trajectory for hydrogel delivery systems will be shaped by further personalization of therapeutic interventions, integration of digital monitoring modalities, and sustainable material sourcing. By synthesizing these findings, stakeholders are better positioned to align strategic initiatives with the next wave of growth catalysts in this dynamic sector.
Engage with Associate Director Ketan Rohom to Acquire In Depth Hydrogel Delivery System Analysis and Unlock Actionable Market Insights for Informed Decision Making
To explore the full depth of analysis including segmented demand statistics, proprietary cost models, and regulatory scenario planning, stakeholders are encouraged to reach out directly to Associate Director Ketan Rohom. By engaging with Ketan Rohom, decision-makers will secure tailored guidance on how to integrate hydrogel delivery system insights into their strategic roadmaps. This collaboration offers immediate access to advanced data visualizations, interactive dashboards, and expert consultations designed to accelerate product development timelines and optimize market entry tactics. Partnering with Ketan ensures that organizations maintain a competitive edge through early identification of emerging trends and potential barriers. Contacting the Associate Director will provide customized briefing materials and responsive support, empowering leadership teams to align investments with tangible growth catalysts. Act now to transform insight into action and elevate your position within the dynamic hydrogel drug delivery ecosystem.

- How big is the Hydrogel-based Drug Delivery System Market?
- What is the Hydrogel-based Drug Delivery System Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




