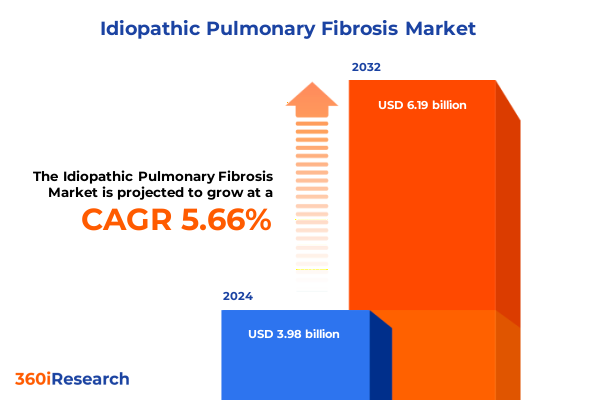
The Idiopathic Pulmonary Fibrosis Market size was estimated at USD 4.19 billion in 2025 and expected to reach USD 4.42 billion in 2026, at a CAGR of 5.71% to reach USD 6.19 billion by 2032.
Navigating the Complexity of the Idiopathic Pulmonary Fibrosis Therapeutic Landscape Through Clinical Challenges Emerging Treatments and Evolving Patient Care Perspectives
Idiopathic pulmonary fibrosis (IPF) represents a progressive and debilitating interstitial lung disorder characterized by irreversible scarring of the pulmonary parenchyma, leading to respiratory failure over time. Despite advances in understanding fibrogenic pathways and immune dysregulation, IPF remains a critical unmet medical need with substantial clinical, economic, and humanistic burdens. This executive summary provides a strategic overview of the current IPF ecosystem, capturing pivotal trends in research, therapeutic development, and patient management approaches.
The introduction contextualizes the market environment, highlighting how demographic shifts, increased diagnostic awareness, and evolving regulatory frameworks shape the therapeutic landscape. It underscores the interplay between novel antifibrotic agents, emerging immunomodulatory strategies, and patient-centered care models. By framing the key drivers influencing IPF treatment paradigms-such as technological breakthroughs in molecular diagnostics, multidisciplinary care delivery, and global supply chain considerations-the narrative sets the stage for deeper analysis. Ultimately, understanding this foundational context is essential for stakeholders seeking to navigate complexities, optimize resource allocation, and anticipate future inflection points in IPF therapeutics.
Unraveling Transformative Technological Molecular and Treatment Paradigm Shifts Redefining Patient Management and Research Focus in IPF
Recent years have witnessed transformative shifts in the IPF landscape, driven by breakthroughs in molecular biology, the advent of precision medicine, and a reinvigorated focus on patient-centric outcomes. Building on established antifibrotic therapies, research institutions and pharmaceutical innovators are exploring novel targets such as epithelial-mesenchymal transition modulators, microRNA regulators, and senolytic pathways. These advances reflect a broader trend toward combination regimens designed to arrest fibrotic progression while preserving lung function and improving quality of life.
Simultaneously, diagnostic sophistication is evolving, with high-resolution imaging and biomarker discovery enabling earlier disease detection and stratified patient enrollment in clinical trials. Digital health technologies, including remote monitoring platforms and artificial intelligence-driven image analysis, now facilitate real-time disease management and proactive intervention. Collectively, these shifts redefine clinical paradigms, requiring stakeholders to adapt their R&D trajectories, align with emerging regulatory guidance on adaptive trial designs, and foster cross-disciplinary collaborations that bridge academic research, clinical practice, and biopharma development strategies.
Assessing the Ramifications of 2025 United States Tariff Policies on Drug Accessibility Supply Chains and Economic Pressures in IPF Therapeutics
The implementation of updated United States tariffs in 2025 has introduced new considerations for IPF drug accessibility, supply chain resilience, and cost management across the value chain. Heightened import duties on active pharmaceutical ingredients and specialized excipients have amplified production expenses for both originators and contract manufacturers. In response, manufacturers are re-evaluating procurement strategies, localizing certain operations, and negotiating alternative supplier agreements to mitigate margin pressures.
From a distribution standpoint, increased logistics costs have influenced inventory stocking patterns at hospital and specialty pharmacies, prompting a rebalancing of on-shore and near-shore warehousing solutions. These dynamics have cascading effects on lead times, cold-chain logistics, and pricing negotiations throughout tender processes. Meanwhile, healthcare providers and payers face the challenge of maintaining patient affordability while contending with reimbursement frameworks that may not yet reflect the impact of tariff-induced cost escalations. Navigating this evolving tariff environment demands agile operational planning, proactive stakeholder communication, and robust scenario modeling to safeguard patient access to essential IPF therapies.
Extracting Actionable Insights From Therapeutic Class Administration Channels Distribution Methods and End User Applications in IPF Care Settings
A nuanced understanding of market segmentation illuminates the multifaceted nature of IPF therapy uptake and patient care preferences. An analysis based on therapeutic class distinguishes the established antifibrotics-namely nintedanib and pirfenidone-from immunosuppressants, which encompass corticosteroids such as methylprednisolone and prednisone as well as immunomodulators including azathioprine and mycophenolate mofetil. This segmentation underscores divergent treatment philosophies, from targeting fibrogenic pathways to tempering dysregulated immune responses.
Equally informative is the scrutiny of route of administration, which spans inhalation methods that enhance localized drug delivery, injectable formulations favored for rapid systemic exposure, and oral dosage forms that maximize patient convenience. Distribution channel evaluation reveals that hospital pharmacies continue to dominate initial prescribing patterns, while online and retail pharmacies expand patient reach through convenient home delivery options and digital prescription management. Specialty pharmacies, in turn, provide critical adherence support and reimbursement navigation for high-cost therapies. Lastly, end user segmentation highlights the diversity of care settings, encompassing ambulatory care centers focused on outpatient management, home healthcare facilities delivering community-based services, acute and chronic care hospitals, and specialty clinics offering concentrated expert care. These segmentation insights offer strategic clarity for tailoring engagement models, optimizing channel mix decisions, and aligning product portfolios with provider and patient needs.
This comprehensive research report categorizes the Idiopathic Pulmonary Fibrosis market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Therapeutic Class
- Route Of Administration
- Distribution Channel
- End User
Illuminating Regional Variations in Idiopathic Pulmonary Fibrosis Treatment Adoption Clinical Infrastructure and Market Dynamics Across Americas EMEA and Asia-Pacific
Regional profiles reveal divergent trajectories in IPF treatment adoption, influenced by healthcare infrastructure maturity, reimbursement environments, and clinical practice norms. In the Americas, robust clinical trial networks and well-established payer systems facilitate rapid uptake of antifibrotic regimens, though affordability challenges persist in underserved communities. The United States leads in new drug approvals and precision medicine initiatives, while Canada and Latin America regions are expanding access through public-private partnerships and patient assistance programs.
Across Europe, the Middle East, and Africa, heterogeneity in regulatory pathways shapes market entry timelines and pricing negotiations. Western Europe’s centralized approval processes and value-based reimbursement models enable predictable launch frameworks, whereas emerging markets rely on adaptable licensing schemes and tiered pricing arrangements. In the Asia-Pacific region, an aging population and heightened interest in respiratory health are driving growth in both branded and generic segments. Japan and Australia exhibit high rates of diagnostic adoption and multidisciplinary care protocols, while Southeast Asian and Oceanian nations are strengthening post-marketing surveillance and enhancing physician education to improve early disease recognition and therapeutic adherence.
This comprehensive research report examines key regions that drive the evolution of the Idiopathic Pulmonary Fibrosis market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Highlighting Strategic Partnerships Pipeline Advancements Competitive Collaborations and Market Positioning by Leading Biopharmaceutical Companies in IPF Field
Leading companies in the IPF domain are deploying a blend of strategic collaborations, targeted pipeline initiatives, and commercial innovations to reinforce their market positions. Established pharmaceutical players are forging alliances with biotech firms specializing in gene editing and fibrosis biology to accelerate next-generation candidate discovery. These partnerships harness complementary expertise, share development risk, and streamline regulatory engagement, particularly in the context of orphan designation and expedited review pathways.
Simultaneously, contract development and manufacturing organizations are scaling capabilities to meet the complex production requirements of biologics and advanced formulations. Publicly traded entities are showcasing robust early-stage pipelines featuring novel small molecules, antibody-drug conjugates, and cell therapy approaches aimed at modulating immune-fibrotic cascades. In parallel, life science service providers are leveraging real-world evidence data platforms and digital patient engagement tools to support value demonstration and improve post-market surveillance. This dynamic competitive landscape underscores the imperative for continuous innovation, agile market entry tactics, and differentiated value propositions to secure a lasting foothold in the IPF space.
This comprehensive research report delivers an in-depth overview of the principal market players in the Idiopathic Pulmonary Fibrosis market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- AstraZeneca PLC
- Boehringer Ingelheim International GmbH
- Bristol-Myers Squibb Company
- F. Hoffmann-La Roche Ltd.
- Galapagos NV
- Gilead Sciences, Inc.
- Glenmark Pharmaceuticals Ltd.
- GNI Group Ltd.
- Horizon Therapeutics PLC by Amgen Inc.
- Johnson & Johnson
- Lupin Ltd.
- Merck & Co., Inc.
- Novartis AG
- Pfizer Inc.
Formulating Strategic and Operational Recommendations for Industry Leaders to Optimize Research Collaborations Manufacturing Agility and Patient Engagement in IPF
Industry leaders must adopt a multi-pronged approach to navigate the evolving IPF ecosystem effectively. First, deepening partnerships across academic centers, clinical research organizations, and patient advocacy groups will expedite translational research and support comprehensive trial recruitment. By aligning on common endpoints and leveraging standardized digital protocols, stakeholders can reduce timeline uncertainties and enhance trial quality.
Operational agility is equally critical; companies should assess supply chain vulnerabilities exposed by tariff fluctuations and diversify sourcing strategies to maintain uninterrupted drug availability. Emphasizing localized manufacturing and strategic stockpiling will bolster resilience while preserving cost efficiency. Moreover, cultivating robust patient support ecosystems-integrating telehealth capabilities, adherence monitoring, and financial assistance-will drive improved treatment persistence and clinical outcomes. Finally, proactive engagement with regulatory bodies to shape adaptive approval frameworks and value-based contracting models will ensure that novel therapies reach patients expeditiously. By executing this cohesive set of initiatives, industry leaders can fortify their competitive edge and deliver meaningful advances in IPF care.
Detailing the Rigorous Multisource Research Methodology Integrating Primary Interviews Secondary Data and Analytical Frameworks Underpinning IPF Market Insights
This report’s findings are underpinned by a rigorous research methodology designed to ensure validity, reliability, and relevance. Primary research included in-depth interviews with clinicians, pharmacoeconomists, supply chain experts, and patient advocates, providing firsthand perspectives on therapeutic challenges and opportunities. Secondary research encompassed a comprehensive review of peer-reviewed journals, clinical trial registries, regulatory filings, and industry conference proceedings, ensuring that all analyses reflect the most current scientific and commercial intelligence.
Quantitative data was synthesized through statistical techniques, including cross-validation of market activity indicators and triangulation against real-world evidence sources. Qualitative inputs were systematically coded and thematically analyzed to extract contextual insights and emerging trends. The analytical framework integrated SWOT (strengths, weaknesses, opportunities, and threats) analyses, value chain assessments, and driver-inhibitor modelling to present a balanced, actionable perspective. Strict adherence to methodological best practices and ethical guidelines ensured data integrity and minimized bias, resulting in robust conclusions that stakeholders can trust to inform strategic decision-making.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Idiopathic Pulmonary Fibrosis market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Idiopathic Pulmonary Fibrosis Market, by Therapeutic Class
- Idiopathic Pulmonary Fibrosis Market, by Route Of Administration
- Idiopathic Pulmonary Fibrosis Market, by Distribution Channel
- Idiopathic Pulmonary Fibrosis Market, by End User
- Idiopathic Pulmonary Fibrosis Market, by Region
- Idiopathic Pulmonary Fibrosis Market, by Group
- Idiopathic Pulmonary Fibrosis Market, by Country
- United States Idiopathic Pulmonary Fibrosis Market
- China Idiopathic Pulmonary Fibrosis Market
- Competitive Landscape
- List of Figures [Total: 16]
- List of Tables [Total: 1431 ]
Consolidating Key Findings Implications and Strategic Considerations to Empower Stakeholders Navigating the Future of IPF Therapies
This executive summary has synthesized critical insights across therapeutic innovation, supply chain dynamics, segmentation nuances, regional adoption patterns, and competitive strategies within the IPF domain. By contextualizing the influence of recent tariff policies, transformative research breakthroughs, and evolving care delivery models, stakeholders gain a holistic view of the challenges and opportunities shaping the market. The segmentation analysis illuminates key entry points for targeted interventions, while regional profiles guide tailored market access and expansion plans.
The convergence of technological advances, patient empowerment, and strategic collaborations heralds a new era in IPF management. Industry participants must remain vigilant to regulatory shifts, dynamic pricing pressures, and the imperative to deliver value beyond clinical efficacy. This conclusion underscores the importance of an integrated approach-one that balances scientific rigor, operational resilience, and patient centricity-to drive sustainable growth and improve outcomes. As the IPF landscape continues to evolve, the insights presented here will serve as a catalyst for informed action and competitive differentiation.
Drive Impactful Decisions and Secure Comprehensive Market Insights by Engaging With Ketan Rohom for Your Custom IPF Research Solutions
The complexities and evolving dynamics of the idiopathic pulmonary fibrosis landscape underscore the importance of having tailored, exhaustive market intelligence at your fingertips. Engaging with Ketan Rohom, Associate Director of Sales & Marketing, will enable you to leverage personalized consultations that align your strategic priorities with in-depth data, expert analysis, and pragmatic recommendations. Through this bespoke engagement, you gain access to the full breadth of our market research report, which delivers granular insights into therapeutic innovations, regulatory implications, competitive activity, and patient-centric considerations.
Partnering with Ketan Rohom ensures you receive timely support in interpreting critical findings, designing customized dashboards, and planning data-driven initiatives that elevate your IPF strategy. His deep understanding of the respiratory therapy sector, combined with our rigorous methodological approach, means you can accelerate decision-making, mitigate risks, and capitalize on emerging opportunities with confidence. To secure this authoritative resource and empower your organization with the clarity and foresight needed to excel, reach out to Ketan Rohom today and transform your strategic planning journey.

- How big is the Idiopathic Pulmonary Fibrosis Market?
- What is the Idiopathic Pulmonary Fibrosis Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




