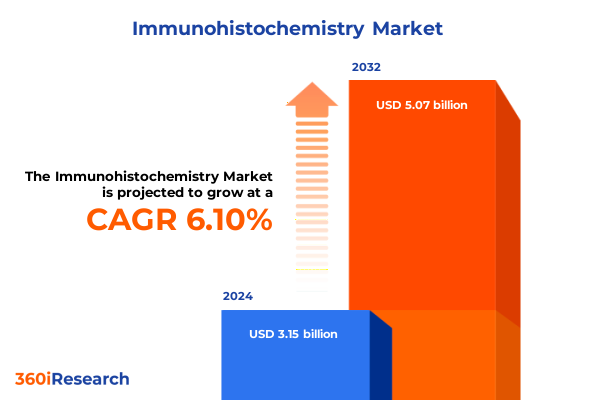
The Immunohistochemistry Market size was estimated at USD 3.33 billion in 2025 and expected to reach USD 3.53 billion in 2026, at a CAGR of 6.16% to reach USD 5.07 billion by 2032.
Unveiling the Promise of Immunohistochemistry: A Comprehensive Exploration of Technological Advancements and Market Dynamics
The field of immunohistochemistry has witnessed transformative growth as advanced molecular techniques converge with digital pathology to create more precise, quantitative, and reproducible diagnostic applications. Over the past decade, technological breakthroughs in antibody engineering, automated staining platforms, and image analysis software have collectively elevated tissue‐based diagnostics to an era of digital integration and clinical relevance. This report opens with an exploration of these underpinning developments, setting the stage for a deeper discussion of the market’s evolving dynamics.
Amid rising demands for personalized medicine and companion diagnostics, the integration of fluorophore‐labeled detection systems and polymer‐based reagents has redefined assay sensitivity and specificity. Concurrently, automated stainers and whole slide imaging systems are reshaping workflow efficiency in high‐throughput laboratories. These milestones have not only broadened the scope of immunohistochemistry applications beyond oncology but also stimulated interest in biomarker discovery across neurology, infectious disease, and autoimmune research.
This introduction frames the strategic relevance of immunohistochemistry in modern healthcare, connecting recent technological innovations to emerging clinical and economic drivers. It provides readers with the critical context needed to appreciate the subsequent analysis of regulatory shifts, tariff impacts, segmentation nuances, regional variances, and key competitive movements that define today’s market landscape.
Examining the Critical Technological Clinical and Regulatory Paradigm Shifts Reshaping Immunohistochemistry Practices Across Research and Diagnostic Applications
The immunohistochemistry environment is undergoing a series of pivotal transformations that are redefining research methodologies and clinical diagnostics. Key among these is the shift from manual, subjective interpretation toward fully automated, digitalized slide processing paired with artificial intelligence–driven image analysis. In parallel, regulatory authorities have updated guidelines for assay validation and quality control, creating a renewed emphasis on standardized protocols and traceable reagent sourcing.
Another crucial shift is the growing convergence of immunohistochemistry and multiplexed tissue analysis techniques. This trend expands diagnostic capabilities by enabling simultaneous detection of multiple biomarkers within a single tissue section. As a result, laboratories can derive more comprehensive phenotypic profiles without increasing sample throughput. Meanwhile, the maturation of digital pathology software and cloud‐based data platforms is facilitating real‐time collaboration among pathologists, accelerating turnaround times for complex cases.
These transformative shifts are also driven by broader healthcare trends, including the rise of precision immuno‐oncology trials and the demand for point‐of‐care devices capable of high‐quality tissue staining. Manufacturers and end users alike are investing in scalable solutions that integrate seamlessly into existing laboratory information systems. As this section demonstrates, the interplay of technology, regulation, and clinical demand is reshaping the foundational landscape of immunohistochemistry.
Analyzing the Far-Reaching Effects of United States Tariff Adjustments in 2025 on Supply Chains Manufacturing Costs and Competitive Positioning
In 2025, the United States implemented revised tariffs on selected imports of specialty chemicals, laboratory instruments, and associated consumables, directly influencing the immunohistochemistry supply chain. This adjustment has led to higher landed costs for imported antibody reagents and staining kits, prompting laboratories and distributors to reassess sourcing strategies. While some suppliers have absorbed tariff increases, others have passed additional charges downstream, resulting in localized price fluctuations for end users.
The tariff environment has further stimulated onshore manufacturing initiatives, as both reagent producers and instrument manufacturers seek to mitigate exposure to import duties. Investments in domestic antibody development facilities and reagent assembly lines have intensified, driven by a desire to secure supply continuity and maintain cost stability. At the same time, certain niche components, such as specialized polymer‐based detection systems, remain heavily reliant on established overseas production hubs, underscoring the ongoing complexity of reshoring efforts.
Importantly, the cumulative impact of these tariff changes has not been uniform across the sector. High‐volume consumables like buffers, mounting media, and chromogenic substrates have faced different tariff classifications compared with advanced imaging scanners or digital pathology software licenses. This uneven application has created pockets of cost sensitivity, encouraging some end users to explore partnership models or multi‐year procurement agreements to lock in favorable terms amid an evolving regulatory backdrop.
Decoding Segment-Level Drivers: In-Depth Insights into Reagents Kits Instruments and Software Service Contributions to the Immunohistochemistry Sector
Segment analysis reveals the nuanced drivers shaping immunohistochemistry demand across reagents and kits, instruments, and software and services. Within the reagents and kits domain, antibodies span monoclonal, polyclonal, and recombinant formats, delivering tailored specificity across diverse tissue targets. Complementing these, buffers and mounting media include antigen retrieval solutions, mounting formulations, and washing buffers to ensure optimal epitope preservation and visualization. Detection reagents further extend capabilities with enzyme‐labeled systems, fluorophore‐labeled compounds, and polymer-based detection platforms that balance sensitivity and multiplexing potential. Meanwhile, substrates and chromogens such as AEC and DAB substrates remain foundational for bright-field applications.
On the instrumentation side, automated stainers-available in both closed and open system formats-are central to throughput and standardization, whereas digital pathology has evolved with whole slide imaging systems and advanced digital pathology software enabling high-resolution image acquisition and analysis. Microscopes, ranging from bright field configurations to confocal and fluorescence platforms, continue to support specialized workflows, particularly in research settings that demand multi-channel visualization.
Finally, the software and services segment encompasses consulting services for protocol optimization, data analysis software for quantitative pathology, maintenance contracts that safeguard instrument performance, and targeted training services designed to upskill laboratory personnel. Together, these segments delineate the complex ecosystem that underpins modern immunohistochemistry applications, illustrating how each facet contributes to overall operational efficiency and diagnostic precision.
This comprehensive research report categorizes the Immunohistochemistry market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Product Category
- Technology
- Specimen Type
- Application
- Indication
- End User
Unraveling Regional Dynamics: How Americas Europe Middle East Africa and Asia Pacific Markets Influence Growth and Adoption in Immunohistochemistry
Regional dynamics in immunohistochemistry are profoundly influenced by differences in healthcare infrastructure, regulatory frameworks, and research funding landscapes. In the Americas, well-established clinical research networks and supportive reimbursement policies have fueled rapid adoption of automated staining platforms and digital pathology integration. This region’s strong cancer research initiatives have also driven demand for high-sensitivity detection reagents and multiplexing capabilities in leading academic and commercial laboratories.
In Europe, the Middle East, and Africa, regulatory harmonization efforts and public-private research collaborations are expanding access to advanced immunohistochemistry solutions. While Western Europe shows steady uptake of turnkey systems in hospital laboratories, Middle Eastern markets are characterized by targeted investments in central pathology facilities. Across Africa, limited infrastructure continues to pose challenges, though portable and cost-effective staining kits have begun to address localized diagnostic needs.
Asia-Pacific has emerged as a dynamic growth hub, supported by expansive pharmaceutical R&D programs and government incentives for biotechnological innovation. Countries such as China, Japan, and South Korea are leading the way in integrating digital pathology across national healthcare systems, while Southeast Asian markets are adopting modular automation solutions to bridge gaps in laboratory capabilities. The divergence in maturity levels across regions underscores the importance of tailored market entry strategies and strategic alliances.
This comprehensive research report examines key regions that drive the evolution of the Immunohistochemistry market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Profiling Leading Industry Players: Competitive Strategies Collaborations Innovations and Market Positioning Shaping the Future of Immunohistochemistry
Key industry players are actively shaping the direction of immunohistochemistry through strategic collaborations, product innovation, and targeted acquisitions. Leading reagent suppliers have introduced recombinant antibody libraries optimized for multiplex assays, bolstering their competitive edge in high-value research and diagnostic segments. Parallel investments in polymer‐based detection platforms have expanded sensitivity thresholds, meeting the rigorous demands of next-generation biomarker studies.
On the instrumentation front, providers of automated staining systems are enhancing their platforms with integrated quality control modules and cloud connectivity to enable remote monitoring and predictive maintenance. Digital pathology vendors continue to refine algorithmic analysis tools, applying machine learning to streamline tumor quantification and immune cell profiling. Simultaneously, service providers offering data analysis software and protocol consulting are broadening their offerings to include AI-powered insights and bespoke training modules.
Mergers and acquisitions have further consolidated competitive positions. Several prominent suppliers have acquired niche analytics firms to integrate advanced imaging software with their existing platforms. These moves not only expand product portfolios but also enhance value propositions for end users seeking end-to-end solutions. As competition intensifies, partnerships between reagent manufacturers, instrument providers, and software developers are likely to accelerate, fostering a more integrated ecosystem.
This comprehensive research report delivers an in-depth overview of the principal market players in the Immunohistochemistry market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Abbott Laboratories
- Abcam plc
- Agilent Technologies, Inc.
- Becton, Dickinson and Company
- Bio-Rad Laboratories, Inc.
- Biogenex Laboratories, Inc.
- Cell Signaling Technology, Inc.
- Danaher Corporation
- Enzo Life Sciences, Inc.
- F. Hoffmann-La Roche Ltd
- Histo-Line Laboratories
- Leica Biosystems Nussloch GmbH
- Merck KGaA
- Miltenyi Biotec B.V. & Co. KG
- Novus Biologicals, LLC
- OriGene Technologies, Inc.
- PerkinElmer, Inc.
- R&D Systems, Inc.
- Santa Cruz Biotechnology, Inc.
- Sigma-Aldrich Co. LLC
- Takara Bio Inc.
- Thermo Fisher Scientific Inc.
- Vector Laboratories, Inc.
- Wako Pure Chemical Industries, Ltd.
- ZenBio, Inc.
Strategic Imperatives for Industry Leadership: Actionable Recommendations to Drive Innovation Market Penetration Operational Efficiency and Long-Term Value
To sustain momentum and capitalize on emerging opportunities, industry leaders should prioritize integrated solution development that bridges reagents, instrumentation, and digital analysis workflows. By consolidating these elements into cohesive platforms, companies can deliver streamlined user experiences, reduce training overhead, and enhance data interoperability. In parallel, forming strategic alliances with clinical research consortia will enable early access to translational cohorts and biomarker validation studies, reinforcing scientific credibility and broadening market reach.
Optimizing supply chains through strategic sourcing partnerships and flexible manufacturing agreements will mitigate risks associated with tariff fluctuations and raw material scarcity. Investing in modular production capabilities closer to key end-user markets can reduce lead times while preserving quality standards. Additionally, offering tiered service models-including remote diagnostics, predictive maintenance, and real-time protocol support-can create recurring revenue streams and deepen customer relationships.
Finally, a continued focus on next-generation assay development, including multiplex immunofluorescence and spatial omics integration, will position organizations at the forefront of personalized medicine. By aligning research roadmaps with emerging clinical trial needs and regulatory priorities, companies can secure strategic partnerships and expedite time-to-market for innovative solutions.
Outlining Comprehensive Research Methodology: Data Sources Analytical Framework Qualitative and Quantitative Approaches Ensuring Robust Insights in Market Analysis
This report’s insights are grounded in a robust research methodology that combines extensive secondary research, expert interviews, and triangulated data analysis. Secondary research involved systematic reviews of peer-reviewed journals, patent filings, regulatory guidelines, and publicly available corporate disclosures to ensure a comprehensive understanding of current technologies and market dynamics. Primary research comprised in-depth discussions with pathologists, procurement officers, academic researchers, and industry executives to capture real-world perspectives and identify unmet needs.
Data analysis employed a multi-layered framework, integrating qualitative assessments with quantitative scoring to evaluate factors such as technology adoption rates, supply chain resilience, and competitive intensity. Regional market profiles were developed through comparative analysis of healthcare infrastructure, reimbursement landscapes, and R&D investment patterns across key territories. Segmentation validation was achieved by correlating product portfolios with end-user application demands, ensuring that reagents, instruments, and service offerings align with specific workflow requirements.
The combination of primary and secondary inputs, along with rigorous data triangulation and validation protocols, underpins the credibility of the findings presented. This methodology ensures that conclusions are not only grounded in objective metrics but are also enriched by qualitative insights from industry stakeholders, fostering a balanced and actionable analysis.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Immunohistochemistry market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Immunohistochemistry Market, by Product Category
- Immunohistochemistry Market, by Technology
- Immunohistochemistry Market, by Specimen Type
- Immunohistochemistry Market, by Application
- Immunohistochemistry Market, by Indication
- Immunohistochemistry Market, by End User
- Immunohistochemistry Market, by Region
- Immunohistochemistry Market, by Group
- Immunohistochemistry Market, by Country
- United States Immunohistochemistry Market
- China Immunohistochemistry Market
- Competitive Landscape
- List of Figures [Total: 18]
- List of Tables [Total: 5406 ]
Concluding Insights on Immunohistochemistry’s Evolution: Synthesizing Technological Trends Market Forces Regulatory Impacts and Strategic Opportunities Ahead
The analysis presented here underscores how technological innovation, regulatory developments, and geopolitical factors are collectively shaping the immunohistochemistry market. Digital transformation initiatives, propelled by automated staining platforms and AI‐driven image analysis, are enhancing diagnostic accuracy and throughput, while updated regulatory standards emphasize reproducibility and traceability. Concurrently, tariff adjustments have recast supply chain strategies, prompting localized manufacturing and strategic procurement arrangements.
Segmentation analysis highlights the diverse drivers across reagents and kits, instrumentation, and software and services, illustrating how each domain contributes to overall operational efficiency and diagnostic precision. Regional insights further reveal that growth trajectories vary significantly between the Americas, Europe Middle East Africa, and Asia-Pacific, necessitating tailored market entry and expansion strategies. Competitive profiling has demonstrated that success hinges on integrated platform development, cross-sector partnerships, and a steadfast focus on next-generation assay capabilities.
Ultimately, the evolving landscape presents both challenges and opportunities for stakeholders across the immunohistochemistry value chain. By embracing strategic imperatives-such as aligning product innovation with clinical trial needs, fortifying supply chain resilience, and leveraging digital pathology integration-industry participants are well positioned to drive the next wave of growth and contribute to improved patient outcomes worldwide.
Engage with Our Expert Analyst to Unlock the Full Immunohistochemistry Market Report and Empower Strategic Decisions Backed by Detailed Insights
In today’s rapidly evolving life sciences landscape, having immediate access to comprehensive market intelligence can be a game changer. To secure the full immunohistochemistry market research report, reach out directly to Ketan Rohom, Associate Director of Sales & Marketing. His expertise will guide you through bespoke options and tailored insights designed to align with your strategic objectives. Whether you are seeking in-depth clarification on regional variations, tariff impacts, segmentation drivers, or competitive dynamics, a conversation with Ketan will ensure you derive maximum value from the analysis.
Don’t miss the opportunity to empower your team with action-ready guidance and robust data. Contact Ketan Rohom to arrange a personalized consultation and obtain the complete report that will support confident decision-making in immunohistochemistry.

- How big is the Immunohistochemistry Market?
- What is the Immunohistochemistry Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




