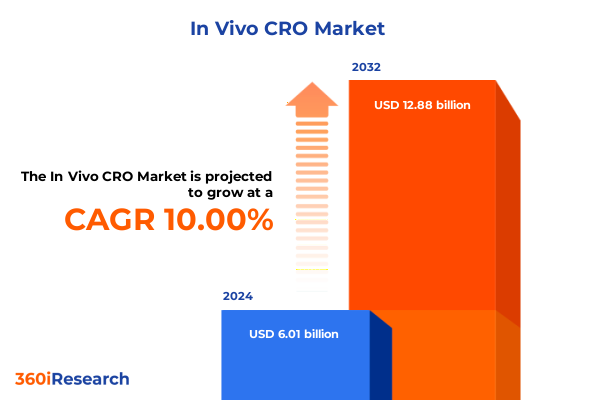
The In Vivo CRO Market size was estimated at USD 6.58 billion in 2025 and expected to reach USD 7.21 billion in 2026, at a CAGR of 10.06% to reach USD 12.88 billion by 2032.
Uncovering the Critical Role of In Vivo CROs in Accelerating Drug Development Through Rigorous Preclinical Research and Strategic Collaboration
The in vivo Contract Research Organization (CRO) sector has emerged as a pivotal force in modern drug development, offering specialized expertise and scalable infrastructure that enable biotech and pharmaceutical entities to navigate the complex preclinical landscape. Historically, companies invested heavily in internal animal facilities and talent to conduct pharmacology, toxicology, and efficacy studies. Over time, tightening budgets, stringent regulatory demands, and the pursuit of operational efficiency have driven a sweeping shift toward outsourcing these critical functions. As drug pipelines become more intricate and time to market tightens, in vivo CROs deliver the agility and scientific rigor necessary to accelerate candidate selection and de-risk the transition to clinical phases. This paradigm shift underscores the sector’s influence on reducing development timelines and optimizing resource allocation for both emerging biotechs and established pharmaceutical giants.
Furthermore, advancements in therapeutic modalities-ranging from small-molecule inhibitors to gene therapies and biologics-have diversified the technical requirements for preclinical evaluation. CROs are now expected to maintain extensive rodent and non-rodent colonies, deploy state-of-the-art imaging and pharmacokinetic platforms, and adhere to evolving Good Laboratory Practice (GLP) and ethical standards worldwide. Coupled with the FDA’s recent endorsements of alternative methods under the 2022 Modernization Act 2.0, which encourages non-animal models while reinforcing robust validation pathways, the role of in vivo CROs has expanded into orchestrating integrated study designs that blend traditional and innovative approaches. This convergence of capability, compliance, and collaboration cements the CRO’s status as an indispensable partner in de-risking and expediting drug discovery.
How Emerging Technologies and Innovative Service Models Are Redefining the In Vivo CRO Landscape to Drive Efficiency and Precision in Drug Discovery
Emerging technologies and evolving client expectations have catalyzed transformative shifts across the in vivo CRO landscape. Artificial intelligence and machine learning now underpin study design optimization, predictive toxicology, and image analysis, enabling more precise data interpretation and reducing reliance on large animal cohorts. Alongside in silico modeling and organ-on-chip systems, these digital innovations complement in vivo workstreams by highlighting potential safety or efficacy signals early in the pipeline. The integration of automated systems for dosing, telemetric monitoring, and health scoring has further elevated throughput while ensuring data integrity and traceability. As a result, leading CROs are forging partnerships with tech providers to co-develop platforms that deliver end-to-end digital workflows, reflecting a broader industry drive toward data-centric, reproducible research.
Concurrently, client demand is shifting toward highly specialized service models that offer full-spectrum support-from study protocol development to regulatory submission packages. CROs are bundling consulting, strategy, laboratory services, and toxicological assessments to furnish turnkey solutions that minimize handoffs and accelerate timelines. This holistic approach is particularly valued by smaller biotech firms that lack in-house regulatory teams and seek guidance through complex approval pathways. Moreover, strategic alliances between CROs and Contract Development and Manufacturing Organizations (CDMOs) are on the rise, signaling a trend toward vertically integrated networks capable of moving candidates seamlessly from discovery to clinical testing. These synergistic models leverage each partner’s core competencies and reflect the industry’s emphasis on collaborative ecosystems that balance agility with technical depth.
Assessing the Far-Reaching Consequences of 2025 United States Trade Tariffs on In Vivo CRO Operations Supply Chains and Cost Structures
In 2025, a series of expanded U.S. trade tariffs targeted high-value preclinical inputs, including specialized laboratory equipment, reagents, imaging systems, and select animal models sourced from China, India, and other key markets. These levies, reaching up to 245% on certain Chinese imports, have materially increased landed costs for in vivo research tools, compelling CROs and their clients to reassess budgeting, procurement strategies, and project timelines. The sudden cost escalation has sparked a strategic pivot toward onshoring initiatives, with domestic breeding facilities and equipment suppliers experiencing surges in demand as organizations seek to insulate themselves from tariff volatility.
The cumulative impact of these measures extends beyond immediate cost pressures. Supply chain disruptions have incentivized alternative sourcing routes, including regional partnerships in Europe and Asia-Pacific jurisdictions unaffected by U.S. tariffs. Some global sponsors are reallocating preclinical work to facilities abroad, where lower import duties and favorable trade agreements help preserve margins. However, this relocation trend presents its own set of regulatory and data integrity challenges, such as harmonizing study protocols across geographies and maintaining consistent animal welfare standards. In response, resilient CROs are deploying hybrid models that blend domestic capacity with offshore labs, augmenting flexibility while retaining critical in-country oversight. These adaptive strategies highlight the sector’s capacity to navigate geopolitical headwinds while safeguarding research continuity.
In-Depth Segmentation Analysis Reveals Diverse Service Types Modalities Indications and User Profiles Shaping the In Vivo CRO Market Dynamics
The diversity of in vivo CRO offerings can be framed across multiple dimensions, each illuminating distinct market dynamics and client priorities. In terms of type, services bifurcate into rodent and non-rodent workstreams, with rodent models predominantly serving high-throughput pharmacology studies, while non-rodent species support regulatory toxicology protocols that demand closer physiological alignment to humans. This delineation enables sponsors to select models optimized for specific endpoints, balancing cost, translatability, and ethical considerations.
Service-type segmentation further differentiates the landscape. Clinical services cater to the late-stage bridging of preclinical safety data to human trials, whereas laboratory services encompass bioanalysis and assay development. Preclinical services encapsulate pharmacokinetics, toxicology, and efficacy testing, forming the core of in vivo CRO activity. Consulting and strategy functions guide study design and regulatory navigation, while specialized regulatory services ensure compliance with evolving local and international standards. Toxicological and safety assessment offerings, often leveraging advanced telemetry and histopathology, provide critical data for Investigational New Drug filings.
Modality-based distinctions also hold sway, with small molecules commanding broad pharmacological screens and dose-ranging studies, and large molecules-biologics, peptides, and oligonucleotides-requiring tailored immunogenicity assays and species-specific safety evaluations. The indication landscape spans cardiovascular, infectious, neurological, oncology, and respiratory disorders, each presenting sub-niches such as coronary artery disease, heart failure, bacterial and viral infections, neurodegenerative and psychiatric conditions, hematological malignancies, solid tumors, asthma, and chronic obstructive pulmonary disease. Finally, end-user segmentation spans academic and research institutions driving early discovery, government and regulatory agencies overseeing compliance, medical device companies testing implants and diagnostics, and pharmaceutical and biotechnology firms seeking partner-driven efficiency. By understanding these interlocking dimensions, stakeholders can align service offerings with specific therapeutic and regulatory imperatives.
This comprehensive research report categorizes the In Vivo CRO market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Type
- Service Type
- Modality
- Indication
- End User
Regional Variations Highlight Distinct Opportunities and Challenges Across the Americas Europe Middle East Africa and Asia Pacific In Vivo CRO Markets
Regional footprints in the in vivo CRO sector reveal differentiated growth drivers and operational nuances across the Americas, EMEA, and Asia-Pacific. In the Americas, advanced infrastructure, robust funding streams, and proximity to leading biotech hubs in the United States and Canada foster a vibrant market for both rodent and non-rodent studies. Regulatory harmonization under agencies like the FDA and Health Canada further streamlines cross-border trial conduct, while recent trade policies have spurred investments in domestic capabilities to offset import tariffs.
Europe, Middle East, and Africa present a mosaic of regulatory frameworks, with the European Medicines Agency’s guidelines converging toward GLP harmonization, even as individual member states maintain localized requirements. Established CRO centers in the U.K., Germany, and France offer deep expertise in oncology and immunology models, while lower-cost Eastern European labs attract sponsors seeking cost efficiencies. Middle Eastern and African markets, still nascent in large-scale preclinical outsourcing, are beginning to attract inbound partnerships spurred by healthcare modernization initiatives and multi-country trial consortia.
Asia-Pacific has evolved into a critical node for in vivo research, propelled by rapidly expanding biotech talent pools in China, India, South Korea, and Japan. Competitive cost structures, coupled with improving quality standards and capacity expansions by regional champions, have drawn both global and domestic sponsors. However, geopolitical tensions and shifting trade tariffs continue to influence site selection and risk mitigation strategies. Across all regions, the imperative to balance quality, compliance, and cost underpins strategic decision-making, with sponsors leveraging multi-regional portfolios to optimize timelines and budgets.
This comprehensive research report examines key regions that drive the evolution of the In Vivo CRO market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Profiling Leading Contract Research Organizations Unveils Strategic Partnerships Innovations and Competitive Differentiators in the In Vivo CRO Sector
Leading in vivo CRO providers are differentiating through strategic partnerships, technology investments, and service diversification. Industry stalwarts such as Charles River Laboratories and Envigo maintain expansive rodent and non-rodent breeding operations, investing in automated health monitoring and digital pathology platforms. These firms have augmented their offerings with bespoke toxicology and safety assessment modules, enabling full IND-enabling packages for global sponsors.
Global consulting and data-analytics giants like IQVIA and ICON have deepened their preclinical footprints by integrating laboratory services with advanced bioinformatics, fostering seamless data exchange from in vivo endpoints to clinical trial design. Their scale enables rapid global deployments, while joint ventures with local partners in Asia-Pacific and EMEA underpin cost-optimized regional access. Meanwhile, midsize specialists such as Medpace have capitalized on nimble operational models, emphasizing client-centric communication and flexible study designs that accommodate emerging modalities like gene therapies and biologics. Additionally, Asia-based champions including WuXi AppTec have accelerated capacity expansions in China and India, responding to localized demand and global sponsor interest in alternative sourcing amid tariff uncertainties.
Emerging players are carving niches in immuno-oncology modeling and neurodegenerative disease platforms, leveraging proprietary animal models and translational biomarkers. Strategic acquisitions, co-development agreements with academic centers, and investments in organ-on-chip interfaces are among the tactics employed to forge competitive advantages. This dynamic competitive landscape underscores the importance of technological leadership, regulatory acumen, and geographic agility in capturing evolving sponsor requirements.
This comprehensive research report delivers an in-depth overview of the principal market players in the In Vivo CRO market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Altogen Labs
- Biocytogen Boston Corp.
- Cellvax, SAS
- Charles River Laboratories International, Inc.
- Crown Bioscience, Inc.
- Eurofins Scientific SE
- Evotec SE
- Explicyte by Immusmol SAS Company
- GEMPHARMATECH LLC
- ICON Plc
- Imavita S.A.S.
- IQVIA Holdings Inc.
- IVRS AB
- JSR Corporation
- Labcorp Drug Development PRIVATE LIMITED
- Medpace, Inc.
- Melior Discovery Inc.
- Noble Life Sciences Inc.
- Parexel International Corporation
- Pharmacology Discovery Services Ltd.
- Pharmaron Beijing Co., Ltd.
- Pharmatest Services Ltd.
- PPD Inc. by Thermo Fisher Scientific, Inc.
- PSI CRO AG
- Syneos Health Inc.
- Syngene International Limited
- Taconic Biosciences, Inc.
- WuXi AppTec, Inc.
- Yecuris Corporation
Strategic Roadmap for Industry Leaders to Optimize In Vivo CRO Collaboration Implement Technology Integration and Navigate Regulatory and Trade Risks
Industry leaders should prioritize building flexible, end-to-end service networks that blend domestic capacity with strategic offshore alliances. By establishing dual-source vendor relationships, companies can mitigate tariff-driven supply risks while preserving timeline commitments. Investing in local breeding facilities and equipment partnerships will further buffer operations against future trade disruptions and ensure uninterrupted study execution.
Harnessing digital platforms and AI-driven analytics across in vivo workflows can unlock efficiency gains and elevate data quality. Organizations should collaborate with technology providers to co-develop predictive toxicology tools and automated health monitoring systems that reduce manual intervention and improve reproducibility. Integrating in silico modeling and organ-on-chip assays early in study design can refine animal usage and enhance translational fidelity.
Developing deep therapeutic-area expertise remains critical. CROs that cultivate specialized teams focused on oncology, neurologic, or infectious disease modeling can command premium value by delivering nuanced protocol designs and biomarker integration. Concurrently, expanding regulatory consulting services will help sponsors navigate evolving global requirements, including emerging alternative-method validations. Cultivating seamless client collaboration-through transparent data sharing portals and dedicated project liaisons-will bolster trust and drive long-term partnerships. Ultimately, a strategic roadmap that balances resilience, innovation, and client-centricity will position organizations for sustainable growth amidst market volatility.
Comprehensive Research Methodology Integrates Primary Interviews Secondary Sources and Data Validation Processes to Ensure Robust Market Insights
This report’s insights derive from a rigorous methodology combining primary and secondary research to ensure depth and accuracy. Primary inputs included in-depth interviews with senior executives from leading CROs, biotech sponsors, regulatory agency representatives, and technology partners, facilitating firsthand perspectives on operational challenges, investment priorities, and emerging service models.
Secondary data was sourced from publicly available regulatory filings, company presentations, industry news platforms, and peer-reviewed journals. Trade policy impacts were analyzed through government tariff schedules, international trade data, and sector-specific commentary. Data triangulation and consistency checks were applied to reconcile disparate sources and validate thematic trends. Quantitative and qualitative findings were subjected to expert review panels to refine hypotheses and confirm sector directionalities.
Confidential-sponsor case studies, integrated with de-identified performance metrics, supplemented macro-level observations. The analytical framework segmented market dynamics across type, service, modality, indication, and end-user dimensions, while regional analyses incorporated regulatory climates, infrastructure capabilities, and geopolitical developments. This comprehensive approach ensures that the report delivers actionable, reliable guidance for decision-makers navigating the evolving in vivo CRO market.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our In Vivo CRO market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- In Vivo CRO Market, by Type
- In Vivo CRO Market, by Service Type
- In Vivo CRO Market, by Modality
- In Vivo CRO Market, by Indication
- In Vivo CRO Market, by End User
- In Vivo CRO Market, by Region
- In Vivo CRO Market, by Group
- In Vivo CRO Market, by Country
- United States In Vivo CRO Market
- China In Vivo CRO Market
- Competitive Landscape
- List of Figures [Total: 17]
- List of Tables [Total: 1749 ]
Converging Trends and Strategic Imperatives Summarize the Evolutionary Trajectory of the In Vivo CRO Market and Guide Future Decision-Making
Collectively, these findings illuminate an in vivo CRO market at the nexus of technological innovation, client-driven specialization, and geopolitical complexity. As sponsors seek to accelerate drug pipelines and de-risk development, the demand for integrated, digitally enabled preclinical services will intensify. Tariff pressures will continue to shape sourcing strategies, underscoring the value of diversified, resilient networks that balance cost efficiencies with regulatory compliance.
Moving forward, organizations that combine specialized disease-area expertise with advanced analytics and alternative-model integrations will stand out in an increasingly competitive landscape. Regional agility-underpinned by onshore capacity and strategic offshore partnerships-will be critical to navigating trade policy uncertainties. By adhering to robust methodological rigor and fostering transparent client engagements, in vivo CROs can solidify their role as essential collaborators in translating scientific breakthroughs into therapeutic realities.
This analysis provides a cohesive roadmap for stakeholders aiming to capitalize on emerging opportunities, mitigate risks, and chart a strategic course in the evolving in vivo CRO domain.
Connect with Associate Director Ketan Rohom to Secure Tailored In Vivo CRO Market Insights Enhance Strategic Planning and Accelerate Growth Outcomes
Ready to gain comprehensive, actionable insights into the dynamic in vivo CRO market? Contact Ketan Rohom, Associate Director of Sales & Marketing, to secure your detailed market research report and empower your strategic decision-making with forward-looking analysis and expert guidance.

- How big is the In Vivo CRO Market?
- What is the In Vivo CRO Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




