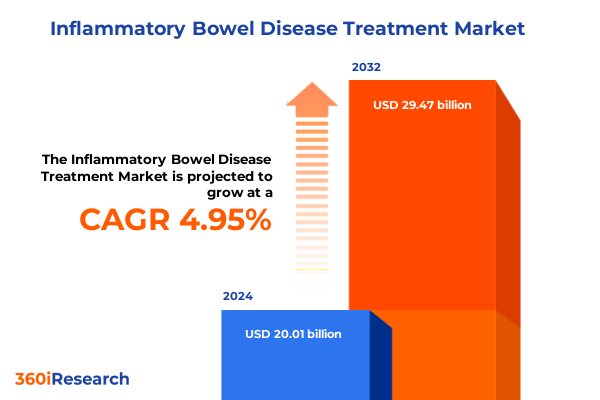
The Inflammatory Bowel Disease Treatment Market size was estimated at USD 20.95 billion in 2025 and expected to reach USD 21.95 billion in 2026, at a CAGR of 4.99% to reach USD 29.47 billion by 2032.
Exploring the Dynamic Emergence of Innovative Approaches Transforming the Treatment Paradigm in Inflammatory Bowel Disease Care
Inflammatory bowel disease continues to pose substantial challenges for clinicians, patients, and industry stakeholders alike as the prevalence of Crohn’s disease and ulcerative colitis steadily intensifies. The complex interplay of genetic predispositions, environmental factors, and immune system dysregulation underscores the imperative for innovative treatment strategies. This report opens with a rigorous exploration of the multifaceted drivers shaping the therapeutic landscape, highlighting the ways in which evolving scientific breakthroughs and shifting patient expectations are redefining standards of care.
Through detailed examination of evolving clinical paradigms and patient-centric considerations, the introduction sets the stage for a deep dive into the core forces propelling progress in inflammatory bowel disease management. It captures the urgency of unmet medical needs, the promise of novel modalities, and the strategic importance of addressing long-term disease burden. By framing the critical context, this section establishes a foundation upon which subsequent analyses of tariffs, segmentation insights, regional nuances, and strategic recommendations are built.
Unveiling Landmark Shifts Driving Next Generation Therapies and Patient-Centric Innovations Within the Inflammatory Bowel Disease Treatment Ecosystem
Over the past several years, the inflammatory bowel disease sector has witnessed unprecedented shifts as novel modalities disrupt legacy treatment algorithms. Advances in genomic medicine and microbiome science are catalyzing the development of gene therapy vectors and microbiota modulators that hold potential to induce disease remission with fewer systemic side effects. Simultaneously, established biologic classes continue to evolve through next-generation antibody engineering, enabling enhanced targeting of integrin receptors and interleukin pathways while reducing immunogenicity.
Moreover, the ascendancy of patient-centric digital tools and remote monitoring solutions is reshaping therapeutic decision making, empowering individuals with real-time disease tracking and personalized adherence support. Together, these transformative shifts underscore a pivotal transition from symptom management toward precision-guided, mechanism-based interventions that promise to deliver more durable outcomes and foster deeper engagement between patients and care teams.
Assessing the Far-Reaching Consequences of Recent 2025 United States Tariffs on Manufacturing Costs Distribution and Access to IBD Therapies
The introduction of new tariff regulations in the United States during 2025 has generated material implications for the production and distribution of both small molecule drugs and biologic agents used in inflammatory bowel disease treatment. Manufacturers have encountered increased import costs for critical active pharmaceutical ingredients, driving heightened attention to supply chain resilience and the localization of manufacturing capabilities. In response, several leading companies have announced strategic partnerships with domestic contract development and manufacturing organizations to secure uninterrupted access to essential components.
Beyond direct cost pressures, these tariffs have catalyzed industry-wide discussions around pricing transparency and patient affordability. Payers and providers are reassessing formulary designs and reimbursement frameworks to mitigate potential out-of-pocket burdens. Consequently, stakeholders are exploring innovative contracting models and outcome-based agreements to ensure sustained access to high-value therapies while navigating the evolving economic logistics precipitated by tariff changes.
Unveiling Core Insights Across Treatment Modalities Administration Routes Formulation Variants Applications Users Distribution Channels Patient Profiles in IBD Care
A nuanced understanding of market segmentation is vital to appreciate how diverse therapeutic categories and patient populations intersect within the inflammatory bowel disease domain. Treatment type analysis highlights four key categories-advanced therapies, biologics, small molecule drugs, and surgical procedures-each representing distinct mechanisms of action and intervention intensity. Advanced therapies encompass frontier technologies such as gene therapy vectors, microbiome modulators, and stem cell therapy, offering potential curative avenues. Biologics include three main classes: integrin receptor antagonists, interleukin inhibitors, and tumor necrosis factor inhibitors, which collectively form the cornerstone of targeted immunomodulation. Meanwhile, small molecule drugs span aminosalicylates, corticosteroids, and immunosuppressants, maintaining foundational roles in inducing and maintaining remission. Surgical procedures range from ostomy creation to intestinal resection and strictureplasty, reflecting the critical role of procedural interventions for refractory disease or complication management.
Complementing this analysis, route of administration segmentation underscores the importance of formulation flexibility and patient preference. Injectable options delivered intravenously or subcutaneously play a pivotal role in high-efficacy regimens, while oral and rectal routes continue to provide convenient, localized delivery for maintenance therapy. Insights into formulation types reveal the strategic value of liquid solutions and suspensions for pediatric dosing, parenteral preparations for rapid bioavailability, and solid dosage forms such as chewable and extended-release tablets to enhance adherence. In addition, applications span Crohn’s disease, indeterminate colitis, and ulcerative colitis, each demanding tailored therapeutic approaches. Consideration of end-user settings-from clinics to home care and hospitals-illuminates treatment access pathways, while distribution channels including hospital pharmacies, online platforms, and retail outlets define supply dynamics. Finally, patient demographics ranging from adult and geriatric populations to pediatric patients inform clinical trial design, dosing regimens, and support services, ensuring that interventions are optimized for diverse cohorts.
This comprehensive research report categorizes the Inflammatory Bowel Disease Treatment market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Treatment Type
- Route Of Administration
- Formulation Type
- Application
- End-User
- Distribution Channel
- Patient Type
Revealing Pivotal Regional Dynamics and Distinctive Market Drivers Shaping IBD Treatment Access and Adoption Trends Across Global Territories
Regional dynamics exert profound influence on the adoption and delivery of inflammatory bowel disease therapies, with each global territory shaped by unique healthcare infrastructures, regulatory frameworks, and patient demographics. In the Americas, robust healthcare spending and well-established reimbursement pathways have facilitated rapid integration of advanced biologic and small molecule interventions. Innovative care models in this region are increasingly embracing telehealth and value-based contracting to improve long-term disease management.
Across Europe, the Middle East, and Africa, heterogeneous regulatory environments and diverse payer systems demand adaptable market entry strategies. Western European countries with streamlined approval processes often lead in early adoption of cutting-edge therapies, while cost containment measures in other EMEA markets drive negotiations on pricing and real-world evidence requirements. Simultaneously, Middle Eastern nations are investing in healthcare infrastructure expansion, creating new access corridors for specialty treatments. In Africa, ongoing efforts to strengthen supply chains and increase diagnostic capabilities are critical to broadening treatment reach.
In the Asia-Pacific region, rapid urbanization and expanding healthcare coverage are stimulating demand for both established and emerging IBD treatments. Innovative payment models in select APAC markets are supporting digital health initiatives and patient engagement platforms. However, variability in regulatory timelines presents both opportunities and challenges for pharmaceutical developers seeking to align product launches with regional policy priorities.
This comprehensive research report examines key regions that drive the evolution of the Inflammatory Bowel Disease Treatment market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Profiling Leading Innovators Disrupting Traditional IBD Therapeutic Spaces and Their Strategic Initiatives Driving Future Treatment Breakthroughs
Leading pharmaceutical and biotech companies are actively advancing portfolios to address the complex needs of inflammatory bowel disease patients through diversified strategic initiatives. International giants are leveraging deep pipeline investments in next-generation biologics targeting novel interleukin and integrin pathways, while also forging partnerships with specialized biotech firms to accelerate development of microbiome-based therapies. Simultaneously, emerging players are carving niche positions by focusing on cell-based and gene-modified approaches, creating collaborative networks that span academic institutions and clinical research consortia.
In addition to R&D activities, major treatment providers are optimizing commercial operations by investing in patient support programs, digital adherence platforms, and real-world evidence studies. These efforts aim to demonstrate long-term value propositions to payers and care providers, reinforcing competitive differentiation. Strategic licensing agreements and mergers have reshaped the competitive landscape, enabling companies to consolidate capabilities in formulation science and global distribution. As regulatory bodies emphasize safety monitoring and post-marketing surveillance, industry leaders are establishing robust pharmacovigilance systems and data-sharing partnerships to ensure therapy optimization and patient safety.
This comprehensive research report delivers an in-depth overview of the principal market players in the Inflammatory Bowel Disease Treatment market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Abbott Laboratories
- AbbVie Inc.
- Abivax
- Amgen Inc.
- Bristol-Myers Squibb Company
- Cipla Limited
- Eli Lilly and Company
- Entera Bio Ltd.
- FutureGen Biopharmaceutical (Beijing) Co., Ltd.
- GlaxoSmithKline PLC
- Ironwood Pharmaceuticals, Inc.
- Johnson & Johnson Services, Inc.
- Merck & Co., Inc.
- Morphic Therapeutic, Inc.
- Nestlé S.A.
- OPKO Health, Inc.
- Pfizer Inc.
- Sanofi SA
- Sun Pharma Limited
- Takeda Pharmaceutical Company Limited
- Teva Pharmaceutical Industries Ltd.
- TScan Therapeutics, Inc.
- UCB S.A.
- Viatris Inc.
- Zealand Pharma A/S
Crafting Actionable Strategies Empowering Industry Leaders to Navigate Complex Treatment Challenges and Capitalize on Emerging IBD Care Opportunities
Industry leaders should prioritize investment in personalized medicine approaches that integrate biomarkers and genomic profiling to enhance treatment precision and patient stratification. By incorporating digital health solutions and remote monitoring tools, manufacturers can foster proactive disease management, improve adherence, and capture valuable real-world insights that support reimbursement dialogues.
Furthermore, companies are advised to strengthen their supply chain resilience by diversifying sourcing strategies and establishing localized manufacturing partnerships to mitigate the impact of policy-driven cost fluctuations. Collaborative engagement with regulatory agencies to co-develop outcome-based contracting models can ensure affordability and access for patients facing financial hurdles. Developing comprehensive education programs for care teams and patients will facilitate smooth adoption of novel modalities, while cross-sector alliances with technology providers can accelerate the integration of artificial intelligence and predictive analytics into clinical decision support.
Detailing Rigorous Research Frameworks and Methodological Approaches Underpinning Comprehensive Analysis of Inflammatory Bowel Disease Treatment Landscape
This analysis is grounded in a rigorous research framework that combines comprehensive secondary intelligence with primary stakeholder interviews. The secondary component incorporates peer-reviewed literature, regulatory filings, real-world data publications, and clinical trial registries to capture evolving therapeutic modalities and policy developments. In parallel, in-depth discussions with key opinion leaders, healthcare practitioners, patient advocacy groups, and industry executives inform nuanced perspectives on unmet needs and adoption barriers.
Data triangulation underpins each insight, ensuring that multiple independent sources corroborate emerging themes. Segmentation logic follows systematic classification protocols across treatment type, administration route, formulation, application, end-user, distribution channel, and patient demographics to enable granular analysis. Quality assurance measures include data validation workshops, methodological audits, and iterative stakeholder feedback loops, reinforcing the credibility and practical relevance of findings.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Inflammatory Bowel Disease Treatment market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Inflammatory Bowel Disease Treatment Market, by Treatment Type
- Inflammatory Bowel Disease Treatment Market, by Route Of Administration
- Inflammatory Bowel Disease Treatment Market, by Formulation Type
- Inflammatory Bowel Disease Treatment Market, by Application
- Inflammatory Bowel Disease Treatment Market, by End-User
- Inflammatory Bowel Disease Treatment Market, by Distribution Channel
- Inflammatory Bowel Disease Treatment Market, by Patient Type
- Inflammatory Bowel Disease Treatment Market, by Region
- Inflammatory Bowel Disease Treatment Market, by Group
- Inflammatory Bowel Disease Treatment Market, by Country
- United States Inflammatory Bowel Disease Treatment Market
- China Inflammatory Bowel Disease Treatment Market
- Competitive Landscape
- List of Figures [Total: 19]
- List of Tables [Total: 2385 ]
Synthesizing Key Findings and Strategic Implications to Conclude Insights That Propel Informed Decision Making Within the IBD Treatment Sector
The synthesis of key findings highlights an industry at the nexus of scientific innovation, economic realignment, and patient empowerment. Breakthroughs in biologic engineering, advanced therapies, and digital health are converging to redefine treatment paradigms, while evolving policy levers such as tariff adjustments and value-based reimbursement reshape commercial strategies. Regional nuances emphasize the importance of adaptive market entry approaches and collaborative stakeholder engagement to ensure broad-based access.
Delivering on the promise of improved patient outcomes will require continued alignment between R&D direction, real-world evidence generation, and supportive care models. The convergence of data-driven insights, flexible contracting mechanisms, and targeted educational initiatives offers a roadmap for organizations seeking to navigate complexity and drive sustainable progress in inflammatory bowel disease care. With the holistic perspective provided in this report, decision makers are well-positioned to translate strategic imperatives into tangible clinical and economic benefits.
Seize This Opportunity to Engage with Our Expert Team for Tailored Insights and Secure Exclusive Access to the Inflammatory Bowel Disease Treatment Report Today
Embark on a strategic journey with our knowledgeable team led by Ketan Rohom, designed to deliver bespoke insights into the rapidly evolving terrain of inflammatory bowel disease treatment. By partnering with us, you will gain exclusive access to an expertly curated report that synthesizes critical trends, examines transformative therapeutic advances, and decodes complex regulatory and economic dynamics. This collaboration ensures that your organization is equipped with the precise intelligence needed to inform product development, align commercial strategies, and optimize patient outcomes.
Connect directly with Ketan Rohom, Associate Director of Sales & Marketing, to explore customized research packages tailored to your unique priorities. Whether you seek deeper understanding of advanced therapies, regional market nuances, or competitor strategies, our experts stand ready to provide actionable guidance and dedicated support. Secure your copy of the comprehensive report today to empower decision makers with clarity, mitigate risks, and capitalize on emerging opportunities in inflammatory bowel disease care.

- How big is the Inflammatory Bowel Disease Treatment Market?
- What is the Inflammatory Bowel Disease Treatment Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




