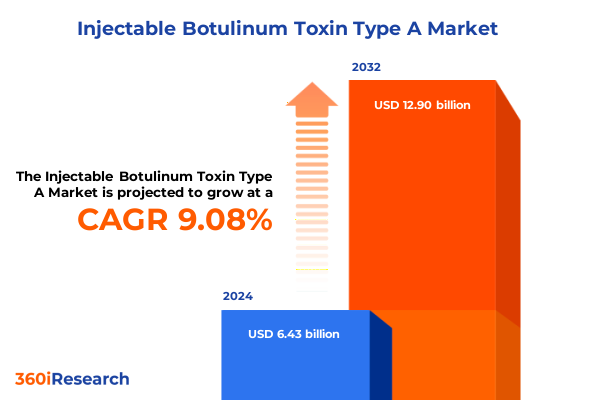
The Injectable Botulinum Toxin Type A Market size was estimated at USD 23.45 billion in 2025 and expected to reach USD 27.34 billion in 2026, at a CAGR of 14.69% to reach USD 61.23 billion by 2032.
Understanding the Emergence and Strategic Importance of Injectable Botulinum Toxin Type A in Modern Therapeutic and Aesthetic Practice
The landscape of injectable Botulinum Toxin Type A has transformed from a niche neuromuscular intervention to a cornerstone in both aesthetic enhancement and therapeutic applications. This evolution is rooted in decades of rigorous clinical research and iterative refinement of formulation and delivery techniques. With escalating patient demand for minimally invasive procedures and expanding therapeutic indications-ranging from chronic migraine management to spasticity treatment-the strategic importance of this biologic agent has never been more pronounced.
As stakeholders navigate evolving regulatory frameworks and heightened safety expectations, they require a holistic understanding of market drivers, technological advancements, and competitive dynamics. This introduction establishes the groundwork for a comprehensive exploration of pivotal shifts, policy influences, segmentation insights, regional contrasts, and leading company strategies. By delving into these dimensions, decision-makers can anticipate emergent opportunities, anticipate challenges arising from policy changes, and chart a resilient course in a rapidly maturing market.
Key Technological Advancements and Regulatory Developments Reshaping the Landscape of Injectable Botulinum Toxin Therapies Across Industries
Recent years have witnessed significant technological breakthroughs in formulation stability, delivery precision, and patient-specific dosing algorithms, culminating in more predictable clinical outcomes and elevated safety profiles. Innovations such as novel excipient systems and advanced device-assisted administration platforms have unlocked new therapeutic potentials while streamlining practitioner workflows. Simultaneously, regulatory bodies have updated guidelines to reflect evolving safety data, prompting manufacturers to adopt more stringent quality controls and post-marketing surveillance measures.
Moreover, the integration of digital tools, including data-driven treatment planning software and telemedicine integration, has democratized access to injectable treatments and fostered more comprehensive patient engagement. This fusion of technology and regulation has catalyzed a shift from one-size-fits-all protocols toward precision-guided interventions that address nuanced patient needs. Consequently, the competitive landscape has tilted in favor of those who leverage both scientific innovation and compliance excellence to differentiate their offerings and forge deeper trust with medical professionals and consumers.
Assessing the Multifaceted Consequences of Recent United States Tariff Policies on the Supply Chain and Accessibility of Botulinum Toxin Type A Treatments
The recent adjustment of United States import tariffs on biologic and peptide-based pharmaceuticals has introduced new complexities into the supply chain for injectable Botulinum Toxin Type A. Manufacturers, distributors, and clinical providers face elevated costs at multiple nodes-from raw material procurement to finished product distribution-which has prompted a reevaluation of sourcing strategies and contractual frameworks. Some organizations have responded by diversifying supplier portfolios and establishing regional manufacturing partnerships to mitigate cost volatility.
In addition, the reconfiguration of tariff codes has accelerated timelines for customs clearance, putting pressure on inventory management practices and clinical scheduling. Forward-thinking companies have turned to integrated logistics solutions that offer real-time tracking and dynamic rerouting to offset potential delays. Meanwhile, stakeholders are engaging proactively with policymakers to advocate for tariff harmonization, emphasizing the broader public health benefits of unhindered access to established and emerging therapeutic uses of Botulinum Toxin Type A.
Unpacking Age Demographics, Distribution Preferences, End-User Behaviors, and Diverse Clinical Applications Shaping Botulinum Toxin Adoption Dynamics
Diverse age cohorts drive unique demand patterns within the Botulinum Toxin Type A market, with individuals aged 25 to 50 years prioritizing subtle aesthetic refinements and preventative treatments, while those above 50 years increasingly seek wrinkle reduction and clinical management of age-associated hyperhidrosis. Conversely, patients below 25 years often engage with treatments for medical indications, reflecting broader acceptance of toxin-based therapies in pediatric and young adult neuromuscular care.
The distribution paradigm has shifted dramatically as offline retail settings maintain their role in established urban and suburban centers, yet online retail channels are gaining traction among digitally native consumers who value convenience, teleconsultation integrations, and home delivery services. In end-user contexts, dermatology clinics leverage specialized expertise for complex facial aesthetics, whereas homecare settings provide flexible administration for chronic disorders. Hospitals remain pivotal for acute and inpatient use, and spas and salons deliver spa-grade cosmetic services that appeal to lifestyle-oriented clientele.
Application-based segmentation reveals a pronounced bifurcation: aesthetic indications such as facial contouring, hyperhidrosis, and non-surgical facelifts are expanding in volume-driven clinics, while medical applications targeting gastrointestinal disorders, muscle spasm alleviation, paralysis management, and multifaceted pain scenarios underscore the toxin’s therapeutic utility across many care settings. Together, these interwoven segments outline a landscape where tailored strategies unlock specific growth zones and optimize resource allocation.
This comprehensive research report categorizes the Injectable Botulinum Toxin Type A market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Age Group
- Distribution Channel
- End-User
- Application
Comparative Regional Insights Revealing Drivers, Challenges, and Competitive Trends in Americas, Europe Middle East & Africa, and Asia-Pacific
Across the Americas, sustained innovation hubs and a dense network of specialized clinics create robust pathways for the adoption of injectable Botulinum Toxin Type A, with regulatory harmonization initiatives supporting cross-border collaborations. In the Europe, Middle East & Africa region, stringent safety standards coexist with dynamic private and public partnerships that accelerate clinical adoption and foster medical tourism, particularly in centers of excellence in dermatology and neurology.
Meanwhile, the Asia-Pacific sphere is defined by rapidly growing consumer awareness, an expanding base of aesthetic clinics, and increasing public sector investments in pain management protocols. Regional market drivers intertwine with distinct reimbursement environments, cultural attitudes toward cosmetic treatments, and variable infrastructural capabilities, necessitating bespoke go-to-market approaches. Collectively, these regional insights underscore the importance of adaptive strategies that align with local regulatory frameworks, patient preferences, and healthcare delivery models.
This comprehensive research report examines key regions that drive the evolution of the Injectable Botulinum Toxin Type A market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Profiling Leading Industry Stakeholders and Innovators Steering Research, Manufacturing, and Commercialization of Injectable Botulinum Toxin Solutions
Market leaders have distinguished themselves through sustained investments in targeted R&D pipelines, focusing on next-generation formulations that minimize diffusion risks and enhance onset consistency. Strategic alliances between biotechnology firms and contract manufacturing organizations have streamlined scale-up timelines and bolstered quality assurance systems. Some companies have also pioneered outcome-tracking platforms that facilitate real-world evidence generation, strengthening adoption among clinical specialists.
In parallel, a cohort of emerging players has introduced biosimilar frameworks and novel peptides that offer alternative biochemical profiles, intensifying competitive dynamics and driving down barriers to entry for new modalities. These innovators often leverage precision marketing tactics, including patient journey analytics and practitioner education series, to carve out differentiated market positions. Ultimately, the interplay between legacy manufacturers, agile biotechs, and specialized service providers is setting the stage for a next wave of diversification in product portfolios and commercial models.
This comprehensive research report delivers an in-depth overview of the principal market players in the Injectable Botulinum Toxin Type A market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- AbbVie Inc.
- Ajinomoto Bio-Pharma Services
- Bio-Med (P) Limited
- Daewoong Pharmaceutical Co., Ltd.
- Evolus, Inc.
- Galderma SA
- Gufic Biosciences Ltd
- Hugel Inc.
- Hugh Source International Ltd.
- Ipsen S.A.
- Medytox Co., Ltd.
- Merz Asset Management Holding GmbH & Co. KG
- Revance Therapeutics, Inc.
- Shanghai Fosun Pharmaceutical (Group) Co., Ltd.
- Sihuan Pharmaceutical Holdings Group Ltd.
Strategic Action Plans Empowering Industry Leaders to Enhance Market Penetration, Optimize Supply Chains, and Propel Therapeutic Innovation
To navigate the complexities of tariff fluctuations, stakeholders should develop multi-tiered sourcing frameworks that integrate local manufacturing options, tiered supplier agreements, and strategic stockholding to preserve margin integrity. Concurrently, investing in predictive analytics for demand forecasting and inventory optimization will minimize the impact of customs delays and supply interruptions, ensuring uninterrupted patient access.
Industry leaders must also prioritize the acceleration of clinical trial designs that incorporate digital monitoring tools, enabling faster data collection and regulatory submissions. By forging alliances with telehealth platforms and academic research centers, organizations can both broaden their clinical reach and facilitate patient enrollment in studies targeting novel indications. Furthermore, targeted practitioner training programs and patient education initiatives will reinforce brand credibility and foster sustained engagement across demographic segments. Together, these recommendations offer a pragmatic blueprint for capturing emergent opportunities while mitigating operational and regulatory headwinds.
Methodological Rigor and Data Integrity Underpinning the Comprehensive Analysis of Injectable Botulinum Toxin Type A Market Dynamics
This analysis synthesizes qualitative and quantitative inputs from a diverse set of sources, including peer-reviewed clinical studies, regulatory filings, proprietary stakeholder interviews, and logistics performance data. Rigorous validation protocols ensure that all third-party information adheres to established standards for reliability, relevance, and recency. Data points are cross-verified through triangulation methods to mitigate bias and confirm contextual accuracy.
Primary research involved structured interviews with leading clinicians, distribution executives, and patient advocacy groups to elicit firsthand perspectives on adoption drivers and pain points. Secondary research encompassed regulatory databases, scientific journals, and publicly available import/export records to map policy influences and competitive actions. Advanced analytical frameworks, leveraging both descriptive and inferential techniques, underlie every insight presented herein, providing a solid foundation for strategic decision-making and scenario planning.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Injectable Botulinum Toxin Type A market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Injectable Botulinum Toxin Type A Market, by Age Group
- Injectable Botulinum Toxin Type A Market, by Distribution Channel
- Injectable Botulinum Toxin Type A Market, by End-User
- Injectable Botulinum Toxin Type A Market, by Application
- Injectable Botulinum Toxin Type A Market, by Region
- Injectable Botulinum Toxin Type A Market, by Group
- Injectable Botulinum Toxin Type A Market, by Country
- United States Injectable Botulinum Toxin Type A Market
- China Injectable Botulinum Toxin Type A Market
- Competitive Landscape
- List of Figures [Total: 16]
- List of Tables [Total: 1113 ]
Synthesizing Key Discoveries and Forward-Looking Perspectives on the Future Trajectory of Injectable Botulinum Toxin Type A Applications
In synthesizing the multifaceted dimensions of injectable Botulinum Toxin Type A-from technological innovations and tariff-driven cost shifts to nuanced segmentation and regional contrasts-this executive summary illuminates the principal forces shaping both current practices and future pathways. The interplay between evolving regulatory landscapes, dynamic demographic preferences, and emerging competitor strategies underscores a market in continuous transformation.
Looking forward, sustained growth will hinge on stakeholders’ ability to integrate digital tools, pursue collaborative research models, and cultivate resilient supply chains that can withstand policy fluctuations. Organizations that align strategic investments with patient-centric outcomes and operational agility will be best positioned to lead in both aesthetic and therapeutic arenas. With these insights in hand, industry participants are equipped to navigate complexity, anticipate disruptions, and chart a course toward enduring success.
Driving Immediate Engagement and Enhanced Decision-Making Through Tailored Access to Comprehensive Botulinum Toxin Market Research Reports
For organizations seeking unparalleled insights into the evolving complexities of injectable Botulinum Toxin Type A, now is the moment to secure a bespoke report tailored to strategic objectives. Engaging with Associate Director Sales & Marketing Ketan Rohom offers a direct path to a comprehensive analysis encompassing regulatory shifts, segmentation nuances, regional dynamics, and competitive intelligence. Through personalized consultation, decision-makers can align market intelligence with investment priorities and product development roadmaps.
Ketan Rohom provides a streamlined process for report acquisition, ensuring that each deliverable addresses the precise contours of business questions around supply chain optimization, therapeutic innovation, and market entry strategies. This call-to-action invites collaboration to refine research deliverables, integrate proprietary datasets, and establish ongoing advisement channels for emerging opportunities. Contacting Ketan Rohom facilitates seamless access to a robust suite of insights that empower cross-functional teams to accelerate growth trajectories, mitigate tariff impacts, and harness demographic trends shaping botulinum toxin adoption.

- How big is the Injectable Botulinum Toxin Type A Market?
- What is the Injectable Botulinum Toxin Type A Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




