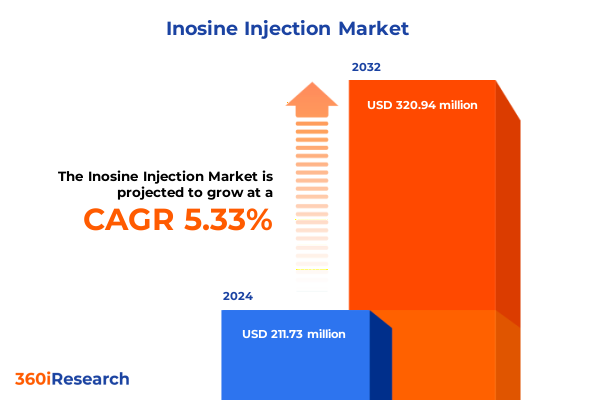
The Inosine Injection Market size was estimated at USD 312.78 million in 2025 and expected to reach USD 338.90 million in 2026, at a CAGR of 8.72% to reach USD 561.77 million by 2032.
Discovering the Emerging Role of Inosine Injection in Modern Healthcare as a Versatile Therapeutic Intervention Across Diverse Clinical Settings
In recent years, clinical interest in Inosine Injection has accelerated substantially as healthcare providers seek novel therapeutic options that can support patient recovery across multiple indications. The pharmacological profile of inosine, known for its role in adenosine metabolism and potential neuroprotective effects, has garnered attention from cardiologists and neurologists alike, expanding its application beyond traditional metabolic therapies. As hospitals and specialty centers incorporate emerging treatment modalities, inosine injection stands out for its potential to modulate inflammatory pathways, reduce oxidative stress, and support tissue repair in settings ranging from ischemic cardiac events to neurodegenerative disorders.
Moreover, the maturation of combination therapies has underscored the value of integrating inosine with other agents to amplify clinical outcomes. Clinicians have reported enhanced patient responsiveness when inosine is administered alongside standard-of-care treatments for arrhythmia management and stroke recovery, reflecting its synergistic potential. This convergence of promising clinical data, coupled with ongoing refinements in injectable formulations that improve bioavailability and stability, has positioned inosine injection as an increasingly viable option for evidence-driven therapeutic regimens. Consequently, investment in formulation innovations and targeted clinical trials continues to rise, reflecting the broader momentum of precision medicine strategies tailored to individual patient profiles.
Examining the Key Transformative Shifts Reshaping the Inosine Injection Marketplace Through Biotech, Digital Health, and Patient-Centered Innovations
The landscape of the Inosine Injection market is undergoing transformative shifts driven by advances in biotechnological innovation and evolving regulatory frameworks. Recent approvals of novel injectable excipients and manufacturing processes have enabled higher purity products, fostering greater clinician confidence in safety and efficacy. Simultaneously, the integration of digital health platforms within hospitals and specialty clinics has facilitated real-time monitoring of patient responses, enabling more precise titration of inosine dosing and personalized treatment adjustments. This digital integration has not only enhanced clinical decision-making but also created new channels for post-market surveillance and outcome-based research.
Concurrently, patient-centric care models have emphasized the importance of minimizing hospital stays while optimizing therapeutic benefit, prompting a reevaluation of inpatient versus outpatient administration of injectable therapies. As a result, online pharmacies and direct vendor sites have gained traction, offering streamlined fulfillment and home administration support. These distribution innovations align with broader healthcare trends emphasizing accessibility and convenience without compromising clinical oversight. In parallel, strategic collaborations between pharmaceutical developers and specialty centers are accelerating clinical trials in neuroprotective applications, reinforcing the market’s pivot from broad metabolic indications toward targeted uses in arrhythmia and stroke recovery.
Analyzing the Comprehensive Impact of U.S. Tariff Policies in 2025 on Inosine Injection Supply Chains, Production Costs, and Regulatory Compliance Dynamics
The landscape of U.S. tariffs on injectable pharmaceutical ingredients has evolved significantly in 2025, influencing both cost structures and supply chain strategies for Inosine Injection. Tariff adjustments on chemical precursors essential to inosine synthesis have prompted manufacturers to reassess sourcing strategies, with several transitioning from high-cost import routes to more diversified procurement from compliant domestic and allied regional suppliers. This realignment has enhanced supply resilience and mitigated the risk of exposure to fluctuating international duties, albeit at the expense of short-term increases in production costs.
Moreover, ongoing dialogue between industry stakeholders and federal authorities has driven policy clarifications that accommodate pharmaceutical supply continuity. Tariff exemptions for active pharmaceutical ingredients under specific trade agreements have eased constraints on large-volume production, paving the way for consistent clinical trial supply and commercial distribution. Nonetheless, contract manufacturers and primary producers continue to allocate resources toward customs compliance and tariff engineering, exploring tariff-shift strategies and origin adjustments to optimize landed cost. These collective efforts underscore the multifaceted impact of U.S. tariff policy on the Inosine Injection value chain.
Unveiling Critical Segmentation Insights That Illuminate Adoption Patterns and Therapeutic Differentiation Across Formulations, End-Users, Distribution Channels, and Dosages
Insightful segmentation underscores how clinical adoption and distribution of Inosine Injection vary across product formulation types, end-user settings, distribution pathways, therapeutic applications, and dosage strengths. Within product types, single-agent formulations have gained early traction in acute arrhythmia management protocols, while combination therapies are carving out a niche in complex neurodegenerative treatment regimens, benefiting from synergistic pharmacodynamics. End users reveal distinct utilization patterns: hospitals continue to dominate high-volume acute care dosing for ischemic events, yet outpatient clinics and rehabilitation and physiotherapy centers are rapidly expanding demand for staged stroke recovery interventions.
When considering distribution channels, hospital pharmacies remain a critical fulcrum for immediate in-hospital dosing, whereas online pharmacies present an increasingly attractive option for follow-on treatment support, leveraging both aggregator platforms and direct vendor sites to serve patients transitioning to home care. Retail pharmacies, encompassing large chains and independent outlets, cater to maintenance dosing scenarios, especially for long-term neuroprotective protocols. In terms of application, cardiology-focused uses for arrhythmia and ischemic disease persist as foundational drivers, while neurology applications in managing neurodegenerative disorders and facilitating post-stroke rehabilitation signal emerging growth corridors. Dosage strength preferences further refine these trends: mid-range concentrations between 50 and 100 mg/mL are standard for acute in-hospital administrations, whereas lower strengths find favor in outpatient monitoring settings and higher concentrations are being piloted in severe neuroprotective clinical trials.
This comprehensive research report categorizes the Inosine Injection market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Product Type
- Application
- Dosage Strength
- End User
- Distribution Channel
Illuminating Distinct Regional Adoption Dynamics for Inosine Injection Across the Americas, EMEA, and Asia-Pacific Reflecting Diverse Healthcare Ecosystems
Regional perspectives reveal diverse trajectories for Inosine Injection adoption and distribution across the Americas, Europe, Middle East & Africa, and Asia-Pacific. In North America, established healthcare infrastructures and streamlined regulatory pathways have facilitated rapid integration of inosine into cardiac and neuroprotective treatment guidelines, supported by extensive clinical collaboration networks spanning academic medical centers and specialty clinics. Latin American markets, while nascent, demonstrate growing interest as regional manufacturers capitalize on trade agreements to reduce dependency on U.S. precursors, fostering localized formulation capabilities.
Across Europe, Middle East & Africa, nuanced regulatory environments and varying procurement models shape market access. Western Europe’s centralized healthcare systems expedite reimbursement discussions, enabling hospitals in Germany, France, and the United Kingdom to pilot expanded uses in ischemic disease management. In contrast, emerging markets in the Middle East and Africa are leveraging public-private partnerships to bolster injectable drug infrastructure and train specialized centers in post-stroke care protocols.
In Asia-Pacific, the interplay between government-led healthcare reforms and private sector investment is underpinning market acceleration. Japan and South Korea benefit from advanced research collaborations that are evaluating inosine’s role in neurodegenerative disease care pathways, while Southeast Asian nations are enhancing cold-chain logistics and digital pharmacy solutions to expand outpatient access. China’s recent policy revisions on import duties and local manufacturing incentives further contribute to an evolving landscape where inosine injection emerges as a strategic therapeutic asset.
This comprehensive research report examines key regions that drive the evolution of the Inosine Injection market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Deciphering the Competitive Terrain of Inosine Injection, Spotlighting Manufacturing Innovations, Strategic Partnerships, and Emerging Biotech Entrants
The competitive environment for Inosine Injection production and distribution features established pharmaceutical manufacturers alongside specialized biotech firms pioneering formulation technologies. Leading contract producers have invested in advanced fermentation platforms and purification systems to maximize inosine yield and reduce impurity profiles. Parallel to this, emerging pharmaceutical developers are filing patent applications for novel delivery mechanisms, including sustained-release injectable depots and combination preparations with synergistic antioxidants and neurotrophic agents.
Strategic alliances are proliferating, with supply agreements linking raw material producers to injectables manufacturers, ensuring seamless upstream-downstream integration. Additionally, select companies are expanding into digital health partnerships to provide patient adherence platforms that track post-discharge administration in rehabilitation centers. These collaborative ventures underscore a shift from commodity-style manufacturing toward value-added service models that bundle product with data analytics and clinical support. As regulatory approvals broaden to encompass new indications, competitive intensity is expected to escalate around quality certifications, distribution exclusivity agreements, and real-world evidence generation.
This comprehensive research report delivers an in-depth overview of the principal market players in the Inosine Injection market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- CR Double-Crane Pharmaceutical Co., Ltd.
- Harbin Pharmaceutical Group Co., Ltd.
- Huazhong Pharmaceutical Co., Ltd.
- Hubei Biocause Pharmaceutical Co., Ltd.
- Pfizer Inc.
- Shandong Xinhua Pharmaceutical Co., Ltd.
- Sichuan Kelun Pharmaceutical Co., Ltd.
- Sinopharm Group Co., Ltd.
- Teva Pharmaceutical Industries Ltd.
- Wanbangde Pharmaceutical Group Co., Ltd.
- Xian-Janssen Pharmaceutical Ltd.
- Zhejiang Huahai Pharmaceutical Co., Ltd.
Implementing Forward-Looking Strategies for Supply Chain Resilience, Digital Access Expansion, and Clinical Differentiation to Drive Market Leadership
Industry leaders should consider forging integrated supply networks that combine domestic and allied regional sourcing of inosine precursors to maintain cost flexibility in light of evolving tariff regulations. Embracing digital distribution channels, including aggregator-driven online pharmacies and direct-to-provider vendor sites, will enhance patient access and support seamless transitions from inpatient to outpatient care. Furthermore, prioritizing clinical trials that evaluate combination therapies and advanced delivery platforms can differentiate portfolios and unlock new formularies in cardiology and neurology.
To capitalize on regional growth disparities, developing tailored market entry strategies-from public-private partnerships in the Middle East & Africa to reimbursement advocacy in Western Europe-will be critical. Leaders should also invest in patient engagement technologies that monitor adherence across retail pharmacy dispensing networks. By aligning product development roadmaps with emerging neuroprotective and ischemic disease treatment protocols, companies can position inosine injection as a flagship offering within broader cardiovascular and neurodegenerative treatment ecosystems.
Outlining a Rigorous Multistage Research Methodology Integrating Expert Interviews, Regulatory Analysis, and Real-World Distribution Data
This research harnessed a multistage methodology combining primary and secondary data sources to ensure a robust and comprehensive analysis. Expert interviews were conducted with clinicians, formulation scientists, and distribution channel stakeholders to capture nuanced insights on treatment protocols, adoption drivers, and logistical constraints. Simultaneously, a systematic review of peer-reviewed publications, regulatory filings, and industry white papers provided context on clinical efficacy, safety profiles, and evolving policy landscapes.
Supplementary data triangulation incorporated information from customs databases, trade agreements, and tariff schedules to quantify the impact of 2025 tariff modifications on the value chain. Distribution channel assessments relied on proprietary pharmacy fulfillment records and digital platform performance metrics, while segmentation and regional analyses were validated through stakeholder roundtables and field surveys in key markets. This integrated approach ensured that conclusions reflect real-world operational considerations and the latest clinical and regulatory developments.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Inosine Injection market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Inosine Injection Market, by Product Type
- Inosine Injection Market, by Application
- Inosine Injection Market, by Dosage Strength
- Inosine Injection Market, by End User
- Inosine Injection Market, by Distribution Channel
- Inosine Injection Market, by Region
- Inosine Injection Market, by Group
- Inosine Injection Market, by Country
- United States Inosine Injection Market
- China Inosine Injection Market
- Competitive Landscape
- List of Figures [Total: 17]
- List of Tables [Total: 1749 ]
Synthesizing Strategic Imperatives and Clinical Drivers That Will Define the Future Trajectory of the Inosine Injection Market
Inosine Injection is poised to remain at the forefront of cardiology and neurology therapeutic protocols as clinical evidence continues to affirm its role in arrhythmia management and neuroprotective strategies. The convergence of advanced formulation technologies, patient-centric distribution models, and strategic supply chain realignments driven by tariff considerations will shape its evolution. Firms that adeptly navigate segmentation nuances-from end-user environments to dosage strengths-and engage proactively with regional regulatory landscapes are best positioned to capture emerging opportunities.
Ultimately, the maturation of partnership-driven service models, which fuse product offerings with digital adherence and clinical support tools, will delineate market leaders from followers. As industry stakeholders align around precision therapy paradigms and outcome-based evidence generation, Inosine Injection is set to exemplify the integration of therapeutic innovation with patient-centric care delivery.
Empowering Your Organization with Tailored Market Research Insights to Drive Growth and Innovation in the Inosine Injection Sector
Elevate your strategic insights and operational foresight with an in-depth exploration of the Inosine Injection market dynamics. Engage directly with our Associate Director of Sales & Marketing, Ketan Rohom, to secure your comprehensive market research report and gain a competitive edge through actionable intelligence.

- How big is the Inosine Injection Market?
- What is the Inosine Injection Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




