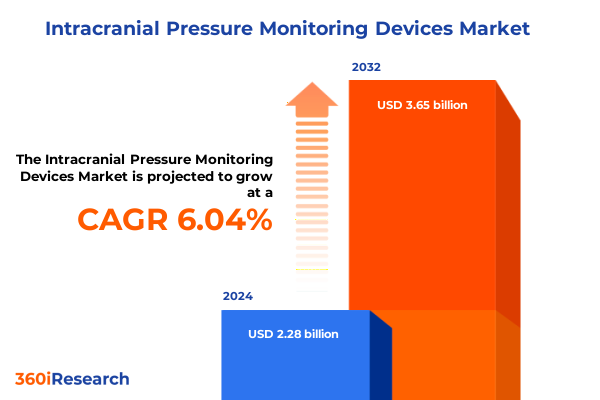
The Intracranial Pressure Monitoring Devices Market size was estimated at USD 2.41 billion in 2025 and expected to reach USD 2.57 billion in 2026, at a CAGR of 6.05% to reach USD 3.65 billion by 2032.
Comprehensive overview of intracranial pressure monitoring highlighting key clinical challenges and emerging innovations redefining neurological care delivery
Intracranial pressure monitoring has evolved into an indispensable component of neurocritical care, providing clinicians with real-time data to inform life-saving interventions. Historically, the invasiveness of traditional monitoring techniques posed significant risks, but ongoing innovation has steadily minimized complications while enhancing signal fidelity. As hospitals and surgical centers strive for precision medicine, the integration of advanced sensor technologies has become pivotal in shaping patient management pathways.
Moreover, rising incidence rates of traumatic brain injury and hydrocephalus worldwide have amplified the demand for reliable intracranial pressure measurement. Emerging noninvasive modalities, such as ocular sonography and transcranial Doppler ultrasound, are gaining traction against the backdrop of regulatory approvals and growing clinical evidence. These developments underscore the critical nature of pressure monitoring in guiding therapeutic decisions and optimizing neurological outcomes.
Furthermore, as healthcare systems navigate cost pressures and evolving reimbursement frameworks, the selection of monitoring solutions balances clinical efficacy with economic sustainability. This introduction sets the stage for an in-depth exploration of the technological breakthroughs, market dynamics, and strategic imperatives that define the current and future landscape of intracranial pressure monitoring.
Innovative technological advancements and regulatory evolutions catalyzing a paradigm shift in intracranial pressure monitoring toward precision and safety
The landscape of intracranial pressure monitoring is undergoing a profound transformation driven by seamless integration of cutting-edge sensor technologies and adaptive regulatory frameworks. Invasive monitoring platforms have been refined through miniaturization of fiber optic and microtransducer sensors, resulting in reduced procedural risk and enhanced accuracy. These enhancements facilitate timely detection of pressure fluctuations in neurosurgical and intensive care environments, reinforcing clinician confidence in critical decision making.
Concurrently, noninvasive techniques are experiencing a renaissance. Innovations such as ocular sonography and tympanic membrane displacement measurements are benefiting from advanced signal processing algorithms, which improve reliability and user adoption outside of traditional intensive care units. Furthermore, regulatory bodies have begun to recognize these methods through streamlined pathways, accelerating their incorporation into clinical guidelines and broadening access to continuous monitoring in lower acuity settings.
In addition, the convergence of digital health platforms and sensor networks is enabling real-time remote monitoring and predictive analytics. Cloud-based data aggregation and machine learning models are poised to anticipate adverse neurologic events before they manifest clinically. Collectively, these transformative shifts are redefining the boundaries of patient-centric monitoring, elevating both the safety profile and diagnostic capabilities of intracranial pressure assessment.
Assessment of the impact of 2025 United States tariffs on intracranial pressure monitoring supply chains, acquisition costs, and healthcare provider procurement
In 2025, the United States introduced a series of tariffs targeting imported medical device components, including fiber optic cables, microtransducer elements, and valve assemblies critical to intracranial pressure monitoring systems. These levies, ranging from ten to twenty percent, have introduced new cost pressures throughout the supply chain. Device manufacturers are navigating increased procurement expenses, prompting them to renegotiate contracts and explore alternative sourcing strategies to mitigate margin erosion.
Moreover, hospitals and ambulatory surgical centers face higher acquisition costs, leading procurement teams to scrutinize total cost of ownership more rigorously. The cumulative impact of these tariffs has also influenced inventory management practices, with many providers opting for leaner stock levels and just-in-time ordering to avoid carrying elevated-price components. As a result, supply chain resilience has become a strategic priority, with stakeholders placing greater emphasis on supplier diversification and nearshoring opportunities.
Furthermore, the tariff landscape has catalyzed discussions around domestic manufacturing incentives, as policymakers consider tax credits and grant programs to spur local production of critical sensor technologies. This evolving regulatory environment has the potential to reshape market dynamics by fostering closer collaboration between medical device companies and government entities. Ultimately, the 2025 tariff policy has served as a powerful catalyst for supply chain innovation and strategic adaptation across the intracranial pressure monitoring ecosystem.
Strategic segmentation insights uncovering the nuanced roles of technology, end user, distribution channel, application, and device type shaping critical care delivery
The market segmentation of intracranial pressure monitoring devices reveals a tapestry of specialized technologies, user environments, distribution pathways, clinical applications, and device form factors that collectively drive innovation and decision making. Technology segmentation distinguishes between invasive platforms, which encompass fiber optic sensors renowned for high-precision readings, microtransducer probes valued for their reliability, and strain gauge devices offering robust signal stability, and noninvasive modalities such as ocular sonography, transcranial Doppler ultrasound, and tympanic membrane displacement methods that provide less risky monitoring alternatives.
End user segmentation spans academic research institutes, where government and private labs push the boundaries of sensor science; ambulatory surgical centers, including general surgery centers and neurology clinics, which leverage real-time monitoring to streamline surgical workflows; and hospitals, with ICUs and dedicated neurosurgery centers relying on continuous measurement to drive patient-centric protocols. Distribution channel segmentation differentiates between direct sales models, which allow for tailored customer engagement, distributor networks including OEM distributors and value-added resellers, and e-commerce platforms split between B2B and B2C streams that expand market reach and facilitate rapid procurement.
Application segmentation sheds light on clinical use cases such as hemorrhage management, hydrocephalus treatment, and traumatic brain injury assessment, each demanding specific performance characteristics. Finally, device type segmentation spans a spectrum from epidural sensors and fiber optic probes to intraparenchymal arrays, intraventricular catheters, strain gauge sensors, subarachnoid devices, and subdural implants, underscoring the diverse engineering approaches that cater to distinct anatomical and procedural requirements. Together, these segmentation insights provide a holistic perspective on the nuanced forces shaping technology adoption and purchasing decisions.
This comprehensive research report categorizes the Intracranial Pressure Monitoring Devices market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Technology
- Device Type
- End User
- Distribution Channel
- Application
Comprehensive regional analysis highlighting unique market dynamics, landscapes, and trends across the Americas, Europe Middle East & Africa, and Asia Pacific
Regional dynamics in the intracranial pressure monitoring market are driven by distinct regulatory frameworks, reimbursement models, and healthcare infrastructure maturity. In the Americas, the convergence of advanced clinical protocols and health technology assessments has fostered robust adoption in hospitals and surgical centers. North American providers are increasingly integrating continuous monitoring solutions into neurocritical care pathways, buoyed by favorable Medicare coverage policies and proactive purchasing cycles.
In Europe, Middle East & Africa, harmonized regulatory processes under European directives coexist with diverse national health systems. Leading nations have standardized invasive monitoring guidelines, while emerging markets in the Middle East and Africa are prioritizing capacity building and training programs to implement both invasive and noninvasive monitoring in tertiary care centers. This region’s heterogeneity creates opportunities for modular solutions that adapt to varying resource constraints.
Asia-Pacific presents a landscape of rapid expansion, with key markets such as China, Japan, and India investing heavily in neurosurgical infrastructure. Government initiatives aimed at reducing traumatic brain injury mortality have propelled demand for reliable pressure sensors across hospital networks. Simultaneously, growing private healthcare segments in Southeast Asia are adopting both legacy and next-generation monitoring modalities, catalyzing partnerships with global device manufacturers. Each region’s unique trajectory reinforces the importance of tailored market entry strategies and collaboration with local stakeholders.
This comprehensive research report examines key regions that drive the evolution of the Intracranial Pressure Monitoring Devices market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Insightful evaluation of leading intracranial pressure monitoring companies highlighting strategic initiatives, technology portfolios, and positioning
Market leadership in intracranial pressure monitoring is characterized by a blend of technological prowess, strategic alliances, and robust product portfolios. Established global firms continue to invest in core invasive platforms, refining sensor accuracy and integrating digital interfaces for seamless data visualization. These companies differentiate through end-to-end service offerings that include installation, training, and aftermarket support, solidifying customer loyalty in high-acuity environments.
Simultaneously, emerging players are carving out niches by advancing noninvasive modalities and leveraging software-driven analytics. Partnerships with academic research institutes accelerate validation studies and expand clinical adoption across ambulatory settings. Moreover, collaborations with OEM distributors and value-added resellers extend market reach into underserved regions, while e-commerce channels enable rapid deployment for smaller clinics and research labs.
In addition, companies are forming cross-sector alliances with surgical robotics and neuromonitoring platform providers, creating integrated solutions that enhance procedural workflow and offer consolidated data streams. This multi-pronged competitive landscape highlights the importance of continuous innovation, strategic partnerships, and a customer-centric approach to maintain differentiation and drive sustained growth.
This comprehensive research report delivers an in-depth overview of the principal market players in the Intracranial Pressure Monitoring Devices market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Cerebro Medical
- Codman & Shurtleff, Inc.
- Hanni Medtech
- Integra LifeSciences Holdings Corporation
- Intravent
- Medtronic plc
- Natus Medical Incorporated
- NeuroDrain
- NeuroKinetics, Inc.
- NeuroLogica Corp.
- NeuroMetrix, Inc.
- NeuroPace, Inc.
- Neuros Medical, Inc.
- NeuroWave Systems Inc.
- Orion Medtronic
- RAUMEDIC AG
- Second Sight Medical Products
- Sophysa SA
- Spiegelberg GmbH & Co. KG
- Vittamed Technologies, Inc.
Practical recommendations for industry leaders to optimize technology adoption, manage regulatory complexities, and drive innovation strategies
Industry leaders should prioritize the integration of hybrid monitoring solutions that combine invasive precision with noninvasive convenience. By investing in modular platform architectures, companies can accommodate evolving clinical protocols and streamline upgrade cycles. Furthermore, establishing joint development agreements with academic centers will accelerate validation of emerging technologies and reinforce evidence-based adoption.
To address regulatory complexities, proactive engagement with health authorities is essential. Early alignment on clinical trial designs and real-world evidence requirements will expedite approvals and facilitate market access. Meanwhile, diversifying supply chains through regional manufacturing partnerships can hedge against tariff volatility and minimize lead times for critical components.
Moreover, companies should leverage digital transformation initiatives by deploying cloud-based analytics and remote monitoring services. These capabilities not only enhance patient safety but also open recurring revenue streams through subscription models. Finally, fostering cross-industry collaborations with telehealth providers and surgical robotics firms will create end-to-end neuromonitoring solutions that deliver differentiated value to customers and drive long-term competitive advantage.
Rigorous research methodology delineating data sources, validation techniques, and analytical frameworks underpinning the insights on intracranial pressure monitoring
The research framework underpinning this analysis combines rigorous primary and secondary methodologies to ensure comprehensive and unbiased insights. Primary research involved in-depth interviews with key opinion leaders, neurosurgeons, biomedical engineers, and procurement specialists across major healthcare institutions. These qualitative discussions provided direct perspectives on clinical priorities, pain points, and adoption barriers.
Secondary research encompassed a systematic review of peer-reviewed journals, industry white papers, regulatory filings, and company press releases. Publicly available patent databases and technical specifications were analyzed to map innovation trajectories and benchmark competing technologies. To validate and triangulate data, proprietary databases of device approvals and pricing models were cross-referenced, ensuring accuracy and consistency.
Analytical frameworks included SWOT assessments, five-force competitive analyses, and segmentation matrices that captured market drivers and restraints. All findings underwent peer review by an interdisciplinary panel of technical experts and healthcare strategists, guaranteeing methodological robustness and actionable relevance for stakeholders engaging with the intracranial pressure monitoring domain.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Intracranial Pressure Monitoring Devices market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Intracranial Pressure Monitoring Devices Market, by Technology
- Intracranial Pressure Monitoring Devices Market, by Device Type
- Intracranial Pressure Monitoring Devices Market, by End User
- Intracranial Pressure Monitoring Devices Market, by Distribution Channel
- Intracranial Pressure Monitoring Devices Market, by Application
- Intracranial Pressure Monitoring Devices Market, by Region
- Intracranial Pressure Monitoring Devices Market, by Group
- Intracranial Pressure Monitoring Devices Market, by Country
- United States Intracranial Pressure Monitoring Devices Market
- China Intracranial Pressure Monitoring Devices Market
- Competitive Landscape
- List of Figures [Total: 17]
- List of Tables [Total: 1749 ]
Conclusive synthesis emphasizing the future trajectories, strategic imperatives, and potential opportunities shaping the field of intracranial pressure monitoring
The convergence of invasive precision and noninvasive innovation is charting a new era in intracranial pressure monitoring. Enhanced sensor designs and streamlined regulatory pathways have lowered procedural risks and broadened clinical applicability beyond intensive care units. Meanwhile, the 2025 tariff landscape has reinforced the need for resilient supply chains and strategic sourcing, emphasizing collaboration between manufacturers and policymakers.
As market segmentation reveals diverse technology, end user, and regional nuances, companies must adopt agile strategies to address unique customer requirements. Leading players that harness digital analytics and form cross-sector partnerships will be best positioned to capture emerging opportunities in hemorrhage management, hydrocephalus care, and traumatic brain injury assessment.
Going forward, stakeholders must align on evidence-based protocols, invest in modular platform upgrades, and explore nearshoring to mitigate cost pressures. By doing so, they will not only meet the evolving demands of neurological care but also foster sustainable innovation that ultimately enhances patient outcomes and operational efficiencies.
Secure your comprehensive intracranial pressure monitoring market research report today by reaching out to Ketan Rohom at Associate Director Sales and Marketing
To secure the definitive guide to the intracranial pressure monitoring market and empower your organization with actionable insights, reach out directly to Ketan Rohom, Associate Director Sales and Marketing. By engaging with Ketan, you will receive personalized assistance in tailoring the research to your strategic objectives and gain immediate access to the comprehensive reports, data tables, and exclusive executive briefings that will drive your next phase of investment and innovation.

- How big is the Intracranial Pressure Monitoring Devices Market?
- What is the Intracranial Pressure Monitoring Devices Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




