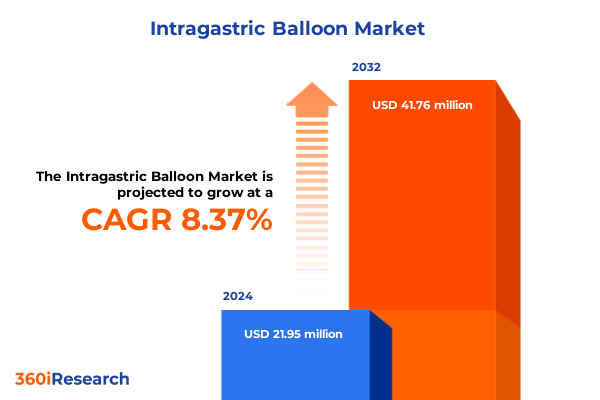
The Intragastric Balloon Market size was estimated at USD 23.49 million in 2025 and expected to reach USD 29.24 million in 2026, at a CAGR of 8.56% to reach USD 41.75 million by 2032.
Navigating the Growing Momentum of Intragastric Balloon Therapy as Obesity Rates Climb and Global Healthcare Innovation Accelerates
The growing prevalence of obesity has emerged as one of the most pressing public health challenges of the twenty-first century, prompting an urgent search for safe, effective, and scalable weight management therapies. Recent estimates from the World Health Organization indicate that over one billion individuals worldwide meet the clinical criteria for obesity, including more than 160 million children, underscoring a trajectory of escalating healthcare burden and comorbidities that spans geographic and socioeconomic boundaries. As bariatric surgery remains invasive and resource-intensive, stakeholders across the healthcare continuum are increasingly drawn to endoscopic and non-surgical interventions that can deliver measurable outcomes with lower procedural risk.
In this context, intragastric balloon therapy has rapidly gained traction as a minimally invasive approach designed to induce early satiety and facilitate sustained behavioral changes. By occupying gastric volume, these devices offer patients and clinicians a non-permanent, reversible modality that complements dietary modifications, physical activity, and pharmacological support. As clinical guidelines evolve to embrace a broader spectrum of obesity treatments, intragastric balloons are transitioning from niche offerings to mainstream tools in the multidisciplinary management of excess weight and its related metabolic complications.
Revolutionary Shifts Are Reshaping the Intragastric Balloon Landscape Through Advances in Patient-Centric Technologies and Treatment Paradigms
Over the past two years, the intragastric balloon landscape has been transformed by the advent of swallowable capsules that eliminate the need for endoscopy. Pioneered by technologies such as the Elipse balloon, these procedureless devices can be ingested as a capsule and autonomously inflated within the stomach, offering patients a streamlined experience without anesthesia or clinical removal procedures. This innovation has resonated with practitioners and end users who seek to expand access to gastric volume reduction techniques outside conventional endoscopy suites, broadening adoption in both urban and resource-constrained settings.
Simultaneously, material science and device engineering advancements have driven differentiation between fluid-filled and gas-filled systems. Fluid-filled balloons continue to lead in patient comfort and adjustability, while gas-filled variants offer reduced weight and enhanced tolerability profiles. Complementing these hardware developments, regulatory approvals across new markets and evolving insurance frameworks have incentivized manufacturers to invest in digital integration-melding telemonitoring platforms, remote patient support, and data analytics to optimize therapy adherence and clinical outcomes.
Examining the Cascading Effects of the 2025 United States Tariffs on Intragastric Balloon Supply Chains and Cost Structures
The introduction of comprehensive United States tariffs in 2025 has imposed significant headwinds for intragastric balloon manufacturers that rely on imported raw materials and components. Elevated duties on polymer formulations, catheter assemblies, and specialized balloon films have tightened cost structures, prompting device makers to reevaluate global sourcing and logistics strategies. Supply chain resilience has become a core strategic imperative, as firms explore nearshoring opportunities and establish partnerships with domestic contract manufacturers to offset cross-border levies and preserve margin integrity.
In parallel, healthcare providers and distributors are adapting commercial models to navigate higher unit costs. Hospitals, bariatric centers, and outpatient clinics have initiated value-based contracting discussions that focus on long-term patient outcomes rather than upfront device pricing, seeking to distribute cost pressures across bundled care pathways. As stakeholders recalibrate procurement protocols, transparent cost reporting and supply chain traceability have emerged as critical enablers to ensure uninterrupted patient access and foster trust among payers and clinical networks.
Unlocking Critical Market Differentiation Insights Through Comprehensive Segmentation of Devices, End Users, Channels, and Applications
Analyzing the intragastric balloon market through a device type lens reveals a dual-track dynamic. Endoscopic systems, subdivided into gas-filled and liquid-filled variants, command established workflows within procedure rooms and offer clinicians precise control over fill volumes. These devices cater to patients seeking a familiar interventional setting, where post-placement adjustments can be performed to align satiety outcomes with individual tolerance. Conversely, the non-endoscopic cohort appeals to those desiring minimal clinical touchpoints, creating a new paradigm for device placement and retrieval that leverages degression valves and soluble materials.
Moving beyond the therapies themselves, end-user segmentation underscores the varied clinical environments in which intragastric balloons find application. Dedicated bariatric centers continue to drive early adoption, capitalizing on comprehensive patient management protocols. Hospitals integrate these devices into multidisciplinary obesity clinics, enabling seamless coordination with nutritionists, endocrinologists, and surgical teams. Meanwhile, outpatient and specialty clinics-whether community-based providers or niche weight-loss practices-are extending service offerings to cater to patient populations seeking convenience and streamlined care journeys.
Distribution channels shape market accessibility and logistical complexity. Hospital pharmacies serve as centralized hubs for procurement and inventory management, ensuring devices meet stringent quality controls and traceability mandates. Retail pharmacies, often located within community settings, provide alternative access points that align with outpatient therapy models, broadening patient options for preoperative optimization or standalone weight loss regimens.
Defining the therapeutic goals further refines the market perspective. In applications focused on preoperative optimization, intragastric balloons assist in reducing perioperative risk by inducing rapid weight loss prior to bariatric surgery. When positioned as a primary weight loss intervention, these devices are integrated into structured lifestyle programs, emphasizing behavioral counseling and nutritional guidance to amplify long-term adherence. Through this multifaceted segmentation, industry stakeholders can tailor product development, market entry strategies, and clinical support frameworks to address diverse patient and provider needs.
This comprehensive research report categorizes the Intragastric Balloon market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Type
- End User
- Distribution Channel
- Application
Illuminating Regional Nuances in the Intragastric Balloon Market Across the Americas, Europe Middle East Africa, and Asia-Pacific
North America continues to represent a significant share of global intragastric balloon adoption, driven by mature healthcare infrastructure, favorable reimbursement policies, and high obesity prevalence. In 2024, the region accounted for over forty percent of total deployments, reflecting robust clinical acceptance and rapid integration into multidisciplinary care pathways. Within the United States, ongoing regulatory engagement and post-market surveillance efforts reinforce patient safety and device efficacy, sustaining clinician confidence and payer support.
In Europe, the Middle East, and Africa, a heterogeneous landscape has emerged. Western European markets with established bariatric and endoscopic service lines are witnessing steady uptake, while select Gulf Cooperation Council countries are leveraging public health initiatives to expand obesity treatment options. Across Africa, gradual infrastructure development and targeted training programs are laying the groundwork for broader access to minimally invasive weight loss therapies.
Asia-Pacific exhibits one of the fastest growth trajectories, bolstered by both public and private sector investment in obesity management and non-surgical weight loss solutions. Notable is the introduction of swallowable gastric balloon capsules in markets such as India, which has catalyzed community-level interest and digital health integration to support remote patient monitoring and teleconsultation models. Meanwhile, supportive insurance frameworks in Australia, Japan, and South Korea further accelerate adoption, signaling a shift toward proactive obesity care across the region.
This comprehensive research report examines key regions that drive the evolution of the Intragastric Balloon market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Profiling the Vanguard of Intragastric Balloon Innovation by Highlighting Key Companies Driving Clinical and Commercial Progress
Among established players, Apollo Endosurgery’s Orbera system remains a benchmark for endoscopic liquid-filled balloons, benefitting from extensive clinical data and a large installed base in hospitals and bariatric centers. ReShape Lifesciences complements this offering with its dual-balloon design, enabling extended gastric occupancy and differentiated patient satisfaction metrics. These incumbents maintain leadership through ongoing post-market studies, iterative device enhancements, and strategic collaborations with key healthcare networks.
Emerging innovators are reshaping competitive dynamics by introducing procedureless and digitally integrated models. Allurion Technologies’ swallowable Elipse balloon has redefined patient convenience, eliminating the need for anesthesia and clinical removal. Obalon Therapeutics leverages gas-filled capsules for multi-balloon deployment, offering graduated satiety control without relying on endoscopic suites. Adjustable systems such as Spatz’s Spatz3 enable clinicians to optimize fill volumes post-deployment, enhancing personalization and extending device dwell times to meet diverse patient goals. Collectively, these companies propel market expansion by catering to novel care delivery models and patient preferences.
This comprehensive research report delivers an in-depth overview of the principal market players in the Intragastric Balloon market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Allurion Technologies Inc.
- Apollo Endosurgery Inc.
- Baronova Inc.
- Boston Scientific Corporation
- Cook Medical LLC
- Districlass Medical S.A.
- Endalis S.A.S.
- Helioscopie Medical Implants
- Johnson & Johnson
- Medicone GmbH
- Medtronic plc
- Olympus Corporation
- ReShape Lifesciences Inc.
- Silimed Indústria de Implantes Ltd
- Spatz FGIA Inc.
Formulating Actionable Strategies for Industry Leaders to Navigate Tariffs, Enhance Accessibility, and Strengthen Supply Chain Resilience
Industry leaders should prioritize strategic localization of manufacturing and assembly to mitigate the impact of evolving trade policies. Establishing nearshore partnerships or domestic contract manufacturing agreements can stabilize supply chains and reduce exposure to cross-border tariffs. This approach not only safeguards cost structures but also enhances responsiveness to regulatory changes and quality management requirements.
Simultaneously, stakeholders can accelerate market penetration by forging value-based contracts that align device reimbursement with longitudinal patient outcomes. By integrating intragastric balloon therapy into bundled care pathways-incorporating nutritional counseling, behavioral therapy, and remote monitoring-manufacturers and providers can demonstrate holistic value, improving payer acceptance and fostering sustained patient engagement.
Finally, embracing digital health platforms and remote support services will be critical to differentiate offerings in a crowded market. Companies that invest in telemonitoring, data analytics, and patient engagement tools can optimize adherence, capture real-world evidence, and refine clinical protocols, ultimately driving better outcomes and reinforcing the case for minimally invasive obesity interventions.
Detailing a Rigorous Research Methodology Combining Secondary Intelligence and Expert Validation to Ensure Robust Market Insights
This research employed a comprehensive two-tiered methodology to ensure the accuracy and relevance of findings. In the secondary phase, extensive literature reviews were performed across peer-reviewed journals, regulatory filings, company disclosures, and reputable news outlets. Proprietary databases and market intelligence platforms complemented these sources, providing granular insights into product pipelines, patent landscapes, and regional regulation dynamics.
Primary validation involved structured interviews with industry experts, including bariatric surgeons, clinical researchers, procurement directors, and device innovators. These engagements offered nuanced perspectives on adoption barriers, clinical protocols, and evolving payer frameworks. Quantitative analyses were subsequently cross-validated through triangulation, reconciling supply chain data, end-user feedback, and public health statistics to underpin robust conclusions.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Intragastric Balloon market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Intragastric Balloon Market, by Type
- Intragastric Balloon Market, by End User
- Intragastric Balloon Market, by Distribution Channel
- Intragastric Balloon Market, by Application
- Intragastric Balloon Market, by Region
- Intragastric Balloon Market, by Group
- Intragastric Balloon Market, by Country
- United States Intragastric Balloon Market
- China Intragastric Balloon Market
- Competitive Landscape
- List of Figures [Total: 16]
- List of Tables [Total: 1113 ]
Concluding Perspectives on the Intragastric Balloon Landscape Emphasizing Strategic Alignment of Innovation, Access, and Patient Outcomes
As obesity continues to fuel demand for minimally invasive therapies, intragastric balloon devices stand at the intersection of clinical innovation and patient-centered care. The convergence of swallowable technologies, adjustable systems, and integrated digital solutions has diversified treatment options, catering to a wider spectrum of patient needs and clinical settings. Moreover, recent tariff-driven realignments in supply chains highlight the importance of resilience and cost transparency for sustained growth.
Looking ahead, successful market participants will be those that blend operational agility with outcome-driven value propositions. By optimizing manufacturing footprints, forging strategic partnerships, and leveraging remote care platforms, the industry is well-positioned to address the global obesity challenge with scalable, evidence-based solutions that enhance both patient outcomes and healthcare efficiency.
Seize Strategic Intelligence on Intragastric Balloon Dynamics Today by Engaging with Our Associate Director for Dedicated Market Research Solutions
To gain unparalleled clarity on the evolving intragastric balloon market and secure a competitive edge, we invite you to collaborate directly with Ketan Rohom, Associate Director of Sales & Marketing. His expertise ensures tailored insights aligned with your strategic objectives, empowering your organization to navigate complex market dynamics and capitalize on emerging opportunities. Reach out today to access the comprehensive market research report that will guide your decision-making and elevate your growth trajectory.

- How big is the Intragastric Balloon Market?
- What is the Intragastric Balloon Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




