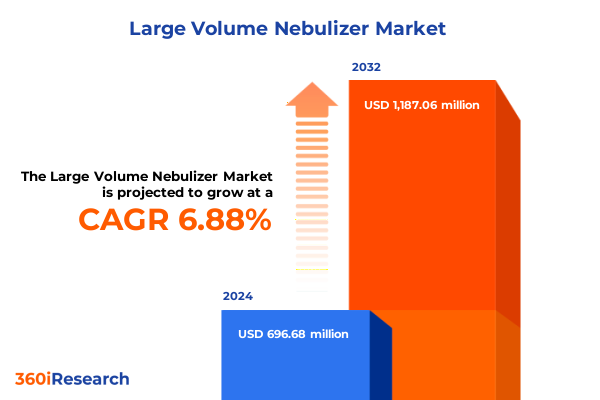
The Large Volume Nebulizer Market size was estimated at USD 743.86 million in 2025 and expected to reach USD 799.26 million in 2026, at a CAGR of 6.90% to reach USD 1,187.07 million by 2032.
Unveiling the Critical Role of Large Volume Nebulizers in Modern Healthcare Amid Rising Respiratory Care Demands and Technological Innovation
The large volume nebulizer has emerged as a cornerstone in respiratory care, bridging the gap between clinical efficacy and patient accessibility. Over recent years, the prevalence of chronic respiratory disorders has intensified, spurring healthcare providers to adopt solutions that ensure reliable drug delivery for vulnerable populations. Unlike portable or handheld devices, large volume nebulizers enable high-dose aerosolized therapy, catering to patients in hospital wards, outpatient centers, and homecare settings where extended treatment sessions are required. Consequently, this category of devices plays a pivotal role in shaping therapeutic outcomes and operational workflows across diverse care environments.
As healthcare delivery models evolve, there is growing recognition of the importance of integrating advanced respiratory technologies within patient care pathways. The demand for seamless, efficient, and safe nebulization has risen in parallel with patient expectations for comfort and convenience. In response, manufacturers have intensified innovation efforts, combining improved aerosol performance with digital monitoring capabilities to enhance adherence and clinical oversight. Ultimately, the large volume nebulizer’s increasing prominence reflects a confluence of epidemiological trends, technological strides, and shifting care paradigms, underscoring the imperative for stakeholders to grasp its strategic significance in modern healthcare.
Examining How Supply Chain Resilience, Technological Integration, and Regulatory Evolution Are Redefining Large Volume Nebulizer Industry Dynamics Today
The landscape of large volume nebulization is undergoing fundamental transformation, driven by the convergence of regulatory reforms, supply chain realignments, and digital integration. In the realm of regulation, new trade measures have prompted industry players to reassess global sourcing strategies. The U.S. Trade Representative finalized adjustments to Section 301 tariffs on respiratory equipment, including increases on respirators and medical gloves slated for 2025, heightening emphasis on reshoring and supplier diversification. Parallel to these shifts, hospitals and clinics are actively collaborating with domestic manufacturers to secure tariff exclusions and expedite import certifications, thereby safeguarding continuity of care.
Simultaneously, technological integration is accelerating, as evidenced by the recent launch of mesh nebulizers equipped with Bluetooth connectivity for remote treatment monitoring. These innovations not only improve particle size consistency but also enable data-driven therapy adjustments based on patient adherence patterns. On the supply chain front, leading device makers are forging partnerships with telehealth platforms and optimizing regional manufacturing footprints to mitigate tariff headwinds and logistic disruptions. Consequently, stakeholders are witnessing a more resilient ecosystem in which clinical teams benefit from integrated digital tools and manufacturers leverage localized operations to uphold service excellence.
Analyzing the Aggregate Consequences of Emerging 2025 Tariff Policies on the United States Large Volume Nebulizer Supply Chain and Cost Structures
In 2025, the cumulative effect of multiple U.S. tariff policies has exerted profound pressure on the large volume nebulizer ecosystem, prompting manufacturers and care providers to implement rapid countermeasures. A universal 10% import levy introduced in April has broadly impacted the cost base for inhalation devices, including compressors, tubing, and ancillary components, compelling procurement teams to reassess vendor relationships and negotiate alternative sourcing arrangements. Layered onto this measure, Section 301 duties on imports from China have escalated specific duties on medical items such as masks, gloves, and syringes to 50%, thereby indirectly influencing the price dynamics of related respiratory equipment.
Healthcare systems reliant on foreign-manufactured nebulizer platforms have grappled with extended lead times and heightened landed costs. As a result, many organizations are accelerating the adoption of in-country assembly lines and co-manufacturing agreements to neutralize tariff-induced cost inflation. Furthermore, major industry titans like GE Healthcare have publicly announced strategic mitigation plans, including the diversification of supply bases and intensified local production, forecasted to alleviate significant tariff burdens by 2026. In parallel, hospitals and outpatient providers are collaborating with policymakers to secure targeted exemptions under the machinery exclusion process, seeking relief for critical care devices. Collectively, these adaptations underscore the sector’s proactive response to the layered trade interventions of 2025.
Deriving Strategic Intelligence from Comprehensive Segmentation of Product Types, Applications, End Users, and Distribution Channels in Nebulizer Markets
The nebuilzer ecosystem is examined through multiple strategic dimensions to reveal nuanced growth drivers and competitive opportunities. When dissecting product typologies, jet nebulizers are categorized into pneumatic jet and venturi configurations, offering robust performance in critical care and acute treatment settings, while mesh devices are subdivided into static and vibrating mesh variants that excel in silent operation and precision dosing. Ultrasonic systems further diversify the portfolio by segmenting into high-frequency and low-frequency models, each tailored to specific aerosol characteristics and patient comfort requirements.
In parallel, therapeutic applications such as asthma, bronchiectasis, chronic obstructive pulmonary disease, and cystic fibrosis shape device selection criteria and influence design enhancements. The contexts in which these interventions are delivered also inform product strategy: ambulatory care centers-including daycare surgery and outpatient treatment units-prioritize rapid setup and throughput, whereas clinics, whether general practice or respiratory specialty, emphasize patient comfort and ease of use. Home care environments extend this paradigm, with home health agencies and personal users demanding portability and minimal maintenance, while hospitals, spanning community health hubs to tertiary referral centers, require scalable systems capable of supporting high-volume treatment loads.
Finally, distribution channels underscore market accessibility and support frameworks. Direct sales channels, through original equipment and third-party distributors, establish foundational relationships with institutional buyers, while hospital pharmacies, both private and public, integrate nebulizers into broader pharmaceutical procurement. The rise of e-commerce marketplaces and manufacturer websites has energized online channels, and traditional retail pharmacies-across chain networks and independent outlets-continue to play a pivotal role in homecare device availability.
This comprehensive research report categorizes the Large Volume Nebulizer market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Product Type
- Application
- End User
- Distribution Channel
Investigating Regional Contrasts in Nebulizer Market Adoption and Infrastructure Across the Americas, EMEA, and Asia-Pacific Economic Zones
Regional dynamics exert profound influence on the trajectory of large volume nebulizer adoption and innovation. In the Americas, a well-established healthcare infrastructure and favorable reimbursement frameworks have catalyzed widespread integration of advanced nebulization platforms across hospitals, ambulatory centers, and homecare networks. This region’s strong portfolio of chronic respiratory disease management programs has created demand for devices with integrated monitoring capabilities, fostering collaborations between device manufacturers and digital health providers.
Across Europe, the Middle East, and Africa, stakeholders navigate a regulatory landscape shaped by the European Medical Devices Regulation, which entered into force in May 2021, mandating stringent safety and performance criteria for medical device marketing. In Western Europe, advanced economies leverage this framework to accelerate deployment of precision nebulizers optimized for aging populations, while emerging healthcare markets in the Middle East and Africa focus on fortifying supply chains and expanding private-sector capacity to meet rising demand.
Asia-Pacific presents a juxtaposition of maturity and growth potential. Leading markets such as China and Japan emphasize cost-efficient mesh and ultrasonic models to address air-quality induced respiratory challenges, whereas Southeast Asia and India witness surging adoption driven by rising disposable income and expanding private hospital networks. In particular, the recent expansion of manufacturing facilities in India underscores the region’s strategic importance for homecare device production, reinforcing the shift toward locally engineered solutions.
This comprehensive research report examines key regions that drive the evolution of the Large Volume Nebulizer market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Assessing Key Corporate Strategies, Innovation Trajectories, and Competitive Postures of Leading Manufacturers in the Large Volume Nebulizer Arena
Market leadership in large volume nebulizers is concentrated among a select cohort of global manufacturers, each deploying differentiated strategies to sustain competitive advantage. Philips Respironics, commanding a significant share of the jet nebulizer segment, emphasizes interoperability and connected care platforms. The recent launch of its Innospire Go 2.0 device, featuring integrated Bluetooth connectivity, exemplifies the integration of real-time usage data with clinical workflows to support personalized therapy monitoring. Omron Healthcare has forged digital health partnerships to embed smart nebulizer functionalities within asthma management applications, broadening its end-user engagement beyond device sales and into chronic care ecosystems.
PARI Respiratory Equipment continues to advance its mesh portfolio, with the eFlow® rapid plus system optimized for targeted drug delivery in clinical trials of inhaled antibiotics. This focus on therapeutic specificity underscores PARI’s strength in specialty pharma collaborations. Drive DeVilbiss Healthcare has pursued geographic expansion through facility upgrades in India, enhancing its capacity to supply cost-sensitive homecare markets and mitigate international tariff exposures. Meanwhile, established names such as GE Healthcare and Allied Healthcare leverage their broad-service networks to deliver turnkey respiratory solutions encompassing device provision, maintenance programs, and operator training.
Collectively, these leading entities invest heavily in R&D to refine aerosol performance, reduce noise levels, and enhance device portability. Their strategic alliances with telehealth platforms, specialized clinics, and pharmaceutical partners underscore a holistic value proposition, fostering recurring revenue streams through consumables, digital subscriptions, and service agreements.
This comprehensive research report delivers an in-depth overview of the principal market players in the Large Volume Nebulizer market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Aeroflow Healthcare
- ATOM Medical Corporation
- B&B Medical Technologies
- Besco Medical Co. Ltd.
- Briggs Healthcare
- Flaem Nuova S.p.A.
- GaleMed Corporation
- GF Health Products Inc.
- Heyer Medical AG
- La Diffusion Technique Française
- Mabis Healthcare Inc.
- Medquip Inc.
- Nidek Medical Products Inc.
- Omron Healthcare Co. Ltd.
- PARI GmbH
- R.S. Medical Inc.
- Rossmax International Ltd.
- Salter Labs
- Teleflex Medical
- Trudell Medical International
- VORTRAN Medical Technology
Outlining Pragmatic Strategic Initiatives for Industry Leaders to Navigate Disruption, Optimize Operations, and Capitalize on Nebulizer Market Opportunities
To navigate escalating trade barriers and rapidly evolving patient needs, industry leaders should prioritize the establishment of diversified supply chain ecosystems. By integrating dual-sourcing strategies, organizations can minimize exposure to tariff fluctuations and geopolitical disruptions. In parallel, investing in regional manufacturing partnerships or in-country assembly operations will enable more agile response to market shifts and local regulatory requirements.
From a product perspective, accelerating the adoption of mesh and ultrasonic technologies with embedded connectivity features can enhance patient adherence and generate actionable clinical insights. Collaborating with telehealth platforms and digital therapeutics providers will extend device value beyond hardware, creating integrated care solutions that differentiate offerings in competitive tender environments. Concurrently, device makers should pursue modular design architectures that facilitate rapid customization for specific therapeutic applications, from asthma management to cystic fibrosis treatment.
Moreover, stakeholder alignment through proactive regulatory engagement is essential. Securing tariff exclusions, participating in machinery exclusion processes, and contributing to MDR conformity discussions will streamline market access. Finally, forging alliances with reimbursement authorities and provider networks will ensure that advanced nebulizer solutions are aligned with evolving care delivery models and funding pathways. This multifaceted approach will empower industry participants to capitalize on emerging opportunities while mitigating risk.
Detailing the Rigorous Multistage Research Framework Underpinning the Nebulizer Market Analysis, Including Data Sources, Validation, and Analytical Procedures
The insights presented in this executive summary are derived from a robust multistage research framework. Initially, extensive secondary research was conducted, encompassing published regulatory enactments, trade commission notices, peer-reviewed technical journals, and manufacturer disclosures. This phase ensured comprehensive mapping of tariff evolutions, device approvals, and technological roadmaps. Subsequently, primary interviews were conducted with key opinion leaders in respiratory therapy, procurement executives within hospital networks, and product innovation specialists to validate secondary findings and capture real-world perspectives.
Market data were triangulated across multiple sources, including customs databases, manufacturing capacity reports, and healthcare expenditure analyses, to authenticate supply chain trends and regional adoption patterns. A rigorous data validation process involving cross-referencing with official trade repositories and regulatory databases was employed to ensure accuracy. Analytical techniques, such as comparative scenario modeling and value-chain cost decomposition, were utilized to elucidate the cumulative impact of tariff policies and segmentation influences. The collective application of these methodologies underpins the reliability of the strategic insights and recommendations outlined herein.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Large Volume Nebulizer market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Large Volume Nebulizer Market, by Product Type
- Large Volume Nebulizer Market, by Application
- Large Volume Nebulizer Market, by End User
- Large Volume Nebulizer Market, by Distribution Channel
- Large Volume Nebulizer Market, by Region
- Large Volume Nebulizer Market, by Group
- Large Volume Nebulizer Market, by Country
- United States Large Volume Nebulizer Market
- China Large Volume Nebulizer Market
- Competitive Landscape
- List of Figures [Total: 16]
- List of Tables [Total: 2385 ]
Consolidating Critical Insights into Nebulizer Market Trends, Technological Advancements, and Strategic Imperatives to Guide Future Decision Making
In synthesis, the large volume nebulizer market is at an inflection point shaped by regulatory shifts, technological breakthroughs, and strategic repositioning. The convergence of rising respiratory disease burdens with evolving care delivery models has elevated demand for high-performance nebulization platforms. At the same time, trade policy dynamics have underscored the necessity of supply chain resilience and local production capabilities.
Segmentation analysis reveals that tailored device architectures, from pneumatic and mesh variants to ultrasonic systems, are essential to meeting diverse clinical and homecare requirements. Regional insights emphasize that market maturation in the Americas, stringent regulatory frameworks in EMEA, and rapid capacity expansion in Asia-Pacific each present distinct opportunities and challenges. Leading companies are responding with innovation-driven strategies, digital integration, and partnerships that extend their value propositions beyond hardware.
Ultimately, stakeholders who adopt integrated approaches-balancing cost management, regulatory compliance, and customer-centric design-will be best positioned to capture growth and reinforce patient outcomes. The strategic imperatives outlined in this summary provide a blueprint for informed decision-making in a complex and dynamic environment.
Engage with Ketan Rohom to Secure Exclusive Market Intelligence on Large Volume Nebulizers and Empower Your Strategic Planning and Growth Initiatives
For authoritative and in-depth insights into the large volume nebulizer market, reach out to Ketan Rohom, Associate Director of Sales & Marketing. Engage with him to explore how this comprehensive analysis can inform your strategic choices, optimize your product road map, and bolster your competitive positioning. He can provide tailored data extracts, address specific questions, and facilitate your procurement of the full market research report. Your next growth opportunity starts with a direct conversation-connect today to unlock exclusive intelligence and actionable market guidance.

- How big is the Large Volume Nebulizer Market?
- What is the Large Volume Nebulizer Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




