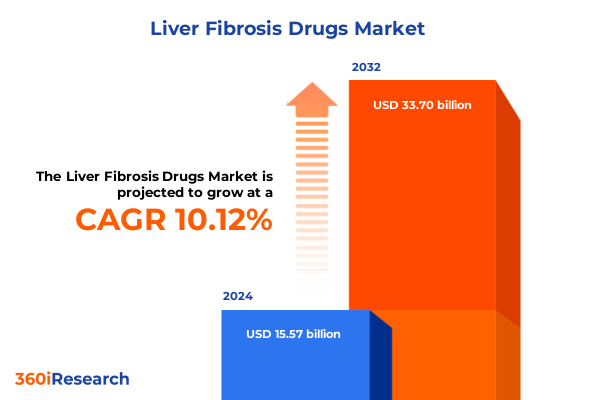
The Liver Fibrosis Drugs Market size was estimated at USD 17.12 billion in 2025 and expected to reach USD 18.83 billion in 2026, at a CAGR of 10.15% to reach USD 33.70 billion by 2032.
Understanding the Evolving Complexity of the Liver Fibrosis Therapeutics Market Amidst Rapid Scientific Advances and Unmet Clinical Needs
Liver fibrosis persists as one of the most pressing public health challenges worldwide, influencing patient outcomes across a spectrum of chronic liver diseases. Driven by common etiologies such as nonalcoholic steatohepatitis, viral hepatitis, and alcoholic liver injury, the progression of fibrosis can culminate in cirrhosis, liver failure, and hepatocellular carcinoma. Despite decades of scientific inquiry, therapeutic options that directly target fibrotic pathways remain limited, and standard-of-care interventions primarily focus on managing underlying causes rather than reversing established scar tissue. Consequently, there is a compelling need for innovative therapies capable of halting or regressing fibrosis at its molecular core.
In recent years, an unprecedented surge in basic and translational research has illuminated the complex network of cellular and extracellular components governing fibrogenesis. Key advances in understanding hepatic stellate cell activation, fibrogenic cytokine signaling, and the role of immune modulation have laid the groundwork for novel drug discovery paradigms. Preclinical models have evolved to more accurately recapitulate human pathology, and biomarker networks are being refined to enable earlier detection and dynamic monitoring of fibrosis progression. The convergence of these scientific breakthroughs with cutting-edge technologies such as high-throughput screening, next-generation sequencing, and machine learning–driven target identification has ushered in a new era of therapeutic exploration.
As stakeholders across the life sciences continuum grapple with the multifaceted challenges of liver fibrosis, strategic alignment between research priorities and clinical needs is more critical than ever. Investment decisions must balance the high attrition rates historically associated with antifibrotic candidates against the profound unmet medical need and potential for transformative clinical benefits. Furthermore, the evolving regulatory landscape, including adaptive approval pathways and biomarker-driven trial designs, underscores the importance of agile development strategies. This section lays the foundation for comprehending the intricate interplay between scientific advances and the persistent unmet needs that define the current liver fibrosis therapeutic arena.
Revolutionary Advances Reshaping the Therapeutic Paradigm for Liver Fibrosis Through Innovation in Biology, Delivery Mechanisms, and Regulatory Landscapes
Over the past decade, the liver fibrosis therapeutic landscape has undergone transformative shifts driven by breakthroughs in biological understanding, technological innovation, and regulatory incentives. The advent of cell and gene therapy platforms has expanded the horizon of what is clinically achievable, with engineered cellular systems poised to modulate the hepatic microenvironment in ways previously thought impractical. At the same time, refinements in monoclonal antibody engineering and recombinant protein expression have yielded next-generation biologics capable of precisely targeting profibrotic signaling molecules, thereby minimizing off-target effects and improving safety profiles.
Parallel to these biological advances, digital health tools and precision medicine frameworks are reshaping clinical development and patient engagement. Real-world data integration and wearable biomarker sensors are facilitating decentralized trial models that enhance patient retention and data granularity, while artificial intelligence–driven analysis accelerates the identification of responder populations. These innovations are complemented by regulatory mechanisms such as breakthrough therapy designations and priority review vouchers, which incentivize companies to pursue high-impact targets and invest in robust biomarker validation strategies.
Integral to these shifts is the rise of collaborative ecosystems, where academia, biotechnology enterprises, and contract research organizations coalesce around shared disease models and open-access data repositories. This trend toward precompetitive collaboration not only accelerates discovery but also de-risks early-stage investments by pooling resources and expertise. As these collective efforts advance, the industry stands at the cusp of a new therapeutic paradigm where combination regimens, adaptive clinical designs, and personalized endpoint selection will define success. The emergent synergies among biology, data science, and regulatory agility underscore the magnitude of change reshaping liver fibrosis drug development.
Evaluating the Compounded Effects of 2025 Tariffs on Cost Structures, Supply Chain Dynamics, and Accessibility in the United States Liver Fibrosis Therapeutics
In 2025, the United States implemented a series of tariffs affecting key raw materials, active pharmaceutical ingredients, and specialty excipients commonly used in liver fibrosis drug production. These levies have compounded manufacturing costs and introduced complexities into established supply chains. Pharmaceutical companies that rely on cross-border procurement have seen margins tighten as cost increases are passed through to downstream partners, including contract manufacturing organizations and bulk chemical suppliers. The resulting pressure on cost structures has prompted manufacturers to reassess supplier diversification strategies and to seek near-shoring opportunities for critical inputs.
As supply chain resilience has become a strategic imperative, many organizations have accelerated initiatives to qualify domestic and regional partners, thereby reducing exposure to tariff volatility. Risk mitigation tactics such as multi-sourcing agreements and dual-site manufacturing have gained traction, but they often require significant capital investments and regulatory approvals. Concurrently, there is mounting interest in in-house capacity expansions for sterile drug products and advanced biologics, as companies aim to internalize critical processes that were previously outsourced to international providers.
The cumulative impact of these tariffs extends beyond production economics; by affecting the landed cost of finished drugs, they also influence pricing negotiations with payers and hospital systems. Some manufacturers have initiated value-based contracting models to preserve market access, offering outcome-linked rebates and bundled payment schemes to offset potential price increases. This strategic recalibration underscores the interconnected nature of procurement, manufacturing, regulatory compliance, and commercialization in a tariff-impacted environment. As stakeholders adapt to these new dynamics, flexible business models and collaborative partnerships will be crucial to sustaining innovation and ensuring patient access to emerging liver fibrosis therapies.
Unearthing Multidimensional Segmentation Insights to Illuminate Patient Pathways, Mechanistic Targets, and Distribution Strategies in Liver Fibrosis Therapeutics
A nuanced understanding of market segmentation provides critical clarity regarding where therapeutic opportunities are most pronounced and which strategies may yield the greatest clinical and commercial returns. From a drug class perspective, the spectrum of modalities includes biologic therapies, cell therapies, gene therapies, and small molecule inhibitors. Within the biologics category, monoclonal antibodies and recombinant proteins serve as cornerstone approaches by targeting extracellular and signaling components of the fibrotic cascade. Small molecule inhibitors, whether derived from natural products or synthetic compounds, offer the flexibility of oral dosing and potential for favorable pharmacokinetic profiles.
Mechanistically, inhibitors of apoptosis signaling kinase 1 and galectin-3 represent distinct pathways for attenuating fibrogenic signaling, while agonists of peroxisome proliferator-activated receptors provide metabolic reprogramming of hepatic cells. PPAR agonists themselves can be further classified based on subtype selectivity-alpha, delta, and gamma-each imparting unique effects on lipid metabolism and inflammatory modulation. Transforming growth factor beta blockers introduce yet another layer of pathway specificity, disrupting a central driver of extracellular matrix deposition.
The route of administration further differentiates therapeutic utility: injectable formats, whether delivered intravenously or subcutaneously, facilitate high-potency and targeted biodistribution, whereas oral regimens offer enhanced patient convenience and adherence. Finally, distribution channels shape market reach and patient experience; hospital pharmacies remain pivotal for acute care settings, online pharmacies enable home delivery models, and retail pharmacies-including both chain and independent storefronts-serve as critical touchpoints for chronic therapy management.
By integrating insights from these four segmentation dimensions, stakeholders can align clinical development strategies with the modalities, mechanisms, administration routes, and channels most relevant to their assets, ultimately maximizing value creation and patient impact.
This comprehensive research report categorizes the Liver Fibrosis Drugs market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Drug Class
- Mechanism Of Action
- Route Of Administration
- Distribution Channel
Analyzing Distinct Regional Dynamics and Growth Drivers Across the Americas, Europe, Middle East & Africa, and Asia-Pacific in Liver Fibrosis Treatment
Regional dynamics in the liver fibrosis landscape are shaped by distinct healthcare infrastructures, regulatory frameworks, and epidemiological profiles. In the Americas, the United States serves as a pivotal innovation hub, propelled by substantial R&D investments, a favorable regulatory environment with expedited pathways, and a sophisticated payer ecosystem that increasingly embraces value-based contracting. Latin American markets, while exhibiting uneven reimbursement landscapes, present growth opportunities through partnerships that expand access to novel therapies and facilitate real-world evidence generation in diverse patient populations.
In the Europe, Middle East, and Africa region, the European Union’s centralized regulatory processes and the European Medicines Agency’s progressive stance on adaptive licensing have created a conducive environment for early entry of breakthrough therapies. Nevertheless, market heterogeneity-from Western Europe’s mature healthcare systems to emerging markets in Central and Eastern Europe-requires tailored strategies for pricing and reimbursement. Meanwhile, Middle Eastern and African nations are gradually strengthening pharmacovigilance frameworks and expanding hepatology specialist networks, laying the groundwork for broader adoption of next-generation antidotes.
The Asia-Pacific region is characterized by a duality of advanced and emerging markets. Japan’s robust biotech infrastructure and proactive orphan drug policies have accelerated the launch of targeted antifibrotic agents, while South Korea and Australia are cultivating strong clinical trial ecosystems. In contrast, populous markets such as China and India, despite rapid generic penetration, are progressively shifting toward innovative therapies as local biopharma firms enhance their capabilities. Cross-border collaborations, technology transfers, and public-private partnerships are increasingly facilitating the introduction of global best practices and high-quality manufacturing standards.
Collectively, these regional insights underscore the importance of customizing market entry and commercial strategies to align with localized regulatory, payer, and patient dynamics, thereby unlocking the full potential of emerging liver fibrosis interventions.
This comprehensive research report examines key regions that drive the evolution of the Liver Fibrosis Drugs market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Examining Leading Competitive Strategies and Pipeline Progress of Key Biotech and Pharmaceutical Companies in the Liver Fibrosis Therapeutics Arena
An examination of the competitive landscape reveals a diverse array of companies pursuing differentiated strategies to capture value in the liver fibrosis domain. Prominent biotechnology firms have leveraged proprietary platforms for antibody engineering and cell therapy development, focusing on early-stage validation of novel targets such as galectin-3 and ASK1. These companies have often secured breakthrough designations, enabling expedited clinical pathways and heightened visibility among key opinion leaders.
Large pharmaceutical corporations have adopted acquisition and licensing models to augment their pipelines, selectively partnering with specialized biotechs to co-develop assets targeting TGF-beta signaling and PPAR pathways. This approach allows established firms to diversify risk across multiple mechanisms of action while capitalizing on existing commercial infrastructures to accelerate market penetration upon approval.
Emerging players are distinguishing themselves by integrating digital therapeutics components and remote monitoring capabilities into their clinical programs, thereby creating holistic treatment solutions that extend beyond traditional pharmacology. This patient-centric emphasis aligns with payers’ growing demand for evidence of real-world effectiveness and adherence data, positioning these innovators favorably for value-based contracting arrangements.
Smaller gene therapy specialists are charting a unique course by focusing on vector optimization and safety profiling, aiming to address specific fibrotic subpopulations with personalized dosing paradigms. By fostering strategic alliances with manufacturing partners and regulatory consultants, these companies are striving to overcome technical barriers and expedite first-in-human studies.
Taken together, these varied competitive approaches highlight the multifaceted nature of the liver fibrosis market, where collaboration, platform differentiation, and patient engagement strategies converge to shape tomorrow’s standard of care.
This comprehensive research report delivers an in-depth overview of the principal market players in the Liver Fibrosis Drugs market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Akero Therapeutics, Inc.
- AstraZeneca plc
- BioLineRx Ltd.
- Boehringer Ingelheim International GmbH
- Bristol-Myers Squibb Company
- FibroGen, Inc.
- Galectin Therapeutics, Inc.
- Galmed Pharmaceuticals Ltd.
- Gilead Sciences, Inc.
- Hepion Pharmaceuticals, Inc.
- Intercept Pharmaceuticals, Inc.
- Inventiva S.A.
- Ionis Pharmaceuticals, Inc.
- Johnson & Johnson Services, Inc.
- Madrigal Pharmaceuticals, Inc.
- Merck & Co., Inc.
- Novartis AG
- Novo Nordisk A/S
- Pfizer Inc.
- Zydus Lifesciences Ltd.
Actionable Strategic Imperatives to Accelerate Innovation, Strengthen Market Positioning, and Navigate Regulatory Complexities in Liver Fibrosis
Industry leaders seeking to navigate the complexities of liver fibrosis drug development must adopt a multi-pronged approach that balances scientific innovation with commercial pragmatism. First, investment in combination regimens that pair antifibrotic agents with metabolic or immunomodulatory therapies can enhance efficacy while mitigating resistance mechanisms. By leveraging existing compounds through novel co-formulation strategies, organizations can de-risk late-stage development and build more robust value propositions for payers and providers.
Second, forging partnerships across the value chain-spanning academic research centers, contract manufacturing organizations, and digital health firms-can accelerate time to clinic and optimize resource allocation. Collaborative alliances enable access to complementary expertise, whether in scalable vector production for gene therapies or in AI-driven trial design for patient stratification. These synergistic relationships can also facilitate shared investment models, reducing the financial burden associated with high-cost clinical trials.
Third, proactive engagement with regulatory authorities and payers is essential to align on evidentiary requirements and pricing frameworks early in the development cycle. Establishing parallel scientific advice pathways and participating in health technology assessment pilot programs can clarify expectations around surrogate endpoints and real-world data integration, thereby minimizing reimbursement delays post-approval.
Finally, embedding patient voices into development strategies through advisory boards and digital feedback loops not only ensures that outcomes align with unmet needs but also strengthens advocacy efforts and accelerates real-world adoption. By translating patient insights into trial endpoints and support programs, companies can demonstrate a commitment to holistic care and differentiate their offerings in a crowded marketplace.
Implementing these strategic imperatives will position industry leaders to deliver transformative therapies, optimize market access, and drive sustainable growth in the evolving liver fibrosis landscape.
Detailing Methodological Framework and Analytical Approaches Underpinning the Rigorous Insights and Data Integrity in Liver Fibrosis Market Research
The methodology underpinning this analysis combines rigorous primary and secondary research, leveraging a structured framework to ensure data integrity and actionable insights. Secondary data were collected from publicly available sources, peer-reviewed literature, company filings, and regulatory databases to establish a robust foundation of scientific, clinical, and commercial intelligence. These sources were critically evaluated for relevance, credibility, and temporal validity to ensure that current trends and developments are accurately captured.
Primary research involved in-depth interviews with key opinion leaders, including hepatologists, clinical trial investigators, and pharmaceutical executives, to validate secondary findings and explore emerging themes. Survey instruments were designed to probe market dynamics, clinical preferences, and strategic priorities, yielding qualitative data that were subsequently triangulated with quantitative indicators. Engagement with supply chain specialists and regulatory consultants provided further context on tariff impacts, manufacturing constraints, and approval timelines.
Data analysis incorporated advanced statistical and analytical approaches, including cross-segment trend mapping, competitive benchmarking, and scenario modeling. Segmentation analyses were performed to elucidate relationships between drug classes, mechanisms of action, administration routes, and distribution channels, while regional modeling accounted for regulatory heterogeneity and payer landscapes. Each analytical module underwent a multi-stage quality assurance process, featuring consistency checks, peer reviews, and validation against external benchmarks.
The resulting insights are presented with the objective of informing strategic decision-making, enabling stakeholders to align investment priorities, optimize development pathways, and anticipate market access challenges. This comprehensive methodology ensures that the report delivers both depth and breadth, translating complex datasets into practical guidance for advancing liver fibrosis therapeutics.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Liver Fibrosis Drugs market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Liver Fibrosis Drugs Market, by Drug Class
- Liver Fibrosis Drugs Market, by Mechanism Of Action
- Liver Fibrosis Drugs Market, by Route Of Administration
- Liver Fibrosis Drugs Market, by Distribution Channel
- Liver Fibrosis Drugs Market, by Region
- Liver Fibrosis Drugs Market, by Group
- Liver Fibrosis Drugs Market, by Country
- United States Liver Fibrosis Drugs Market
- China Liver Fibrosis Drugs Market
- Competitive Landscape
- List of Figures [Total: 16]
- List of Tables [Total: 1590 ]
Synthesizing Strategic Imperatives and Market Realities to Chart the Future Course of Liver Fibrosis Therapeutic Development and Commercialization
The evolving landscape of liver fibrosis therapeutics is defined by a confluence of scientific breakthroughs, regulatory innovations, and strategic realignments. As the industry transitions from single-target paradigms toward multimodal approaches, there emerges a clear imperative to synchronize pipeline development with the nuanced realities of clinical practice and patient heterogeneity. The advent of cell and gene therapies, alongside next-generation biologics and small molecule inhibitors, has significantly broadened the therapeutic toolkit, offering renewed hope for disease modification and improved patient outcomes.
Simultaneously, external factors such as the 2025 United States tariffs have underscored the fragility of global supply chains and the importance of agile manufacturing strategies. Companies that proactively address cost pressures through supplier diversification and localized production will be better positioned to sustain market access and preserve profit margins. In tandem, a deep understanding of segmentation dimensions-encompassing drug class, mechanism of action, administration route, and distribution channel-enables developers to align their assets with the pathways that maximize clinical impact and commercial viability.
Regional dynamics further emphasize the need for tailored market entry and commercialization strategies, as regulatory frameworks and payer expectations vary substantially across the Americas, EMEA, and Asia-Pacific. Collaborative and patient-centric models of care, underpinned by digital health solutions and real-world evidence generation, will continue to reshape adoption curves and value perceptions. Looking ahead, stakeholders must embrace an integrated approach that balances innovation, operational resilience, and evidence generation to navigate the complexities of the liver fibrosis market.
In synthesizing these strategic imperatives and market realities, industry leaders can chart a course that accelerates therapeutic breakthroughs, secures sustainable access, and ultimately transforms the lives of patients living with liver fibrosis.
Engage Directly with Ketan Rohom to Unlock Exclusive Insights and Tailored Guidance for Strategic Deployment of Liver Fibrosis Therapeutics Research
Connect with Ketan Rohom, Associate Director of Sales & Marketing, to acquire the full market intelligence report and gain exclusive access to comprehensive insights, tailored data analyses, and strategic outlooks that will empower your organization to thrive in the evolving liver fibrosis therapeutics landscape. Ketan Rohom’s expertise in translating complex research findings into actionable business strategies ensures you will receive personalized guidance on pipeline prioritization, market entry tactics, and regulatory navigation. Secure your competitive advantage by engaging directly for a detailed briefing that illuminates critical opportunities, risk mitigations, and growth pathways specific to your objectives for 2025 and beyond.

- How big is the Liver Fibrosis Drugs Market?
- What is the Liver Fibrosis Drugs Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




