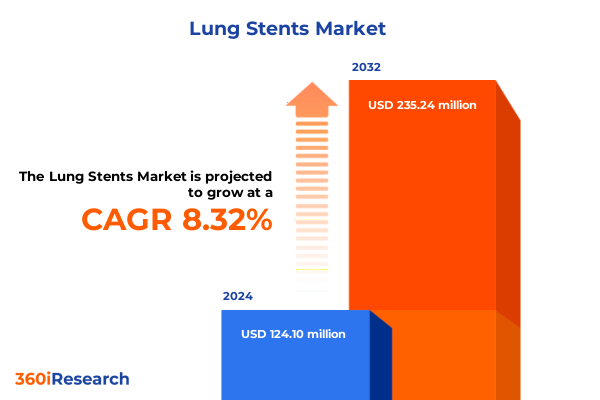
The Lung Stents Market size was estimated at USD 133.35 million in 2025 and expected to reach USD 145.98 million in 2026, at a CAGR of 8.44% to reach USD 235.23 million by 2032.
Understanding the Evolving Lung Stents Landscape and Its Strategic Implications for Healthcare Stakeholders in a Post-Pandemic Era
Understanding the Evolving Lung Stents Landscape and Its Strategic Implications for Healthcare Stakeholders in a Post-Pandemic Era
Over the past decade, the management of central and peripheral airway diseases has undergone a pronounced transformation, driven by advances in device materials, imaging guidance, and procedural techniques. Chronic conditions such as tracheobronchial stenosis and airway obstruction have surged amid aging populations and an increasing burden of malignancies, prompting physicians and healthcare systems to seek more durable, adaptable solutions. As outcomes research continually refines the risk-benefit calculus, lung stents have emerged as indispensable tools for restoring airway patency, palliation of tumor-related complications, and management of congenital or acquired malacia.
In this executive summary, decision-makers will find a cohesive narrative that bridges clinical innovation with market dynamics and policy shifts. The following analysis highlights revolutionary material science breakthroughs, evaluates the ripple effects of tariff measures implemented in early 2025, distills key segmentation insights across product types, indications, patient demographics, and distribution channels, and offers regional perspectives spanning the Americas, EMEA, and Asia-Pacific. For organizations intent on forging competitive differentiation, the insights into leading players, strategic collaborations, and actionable recommendations will serve as a blueprint for aligning product development roadmaps and commercial strategies with evolving stakeholder needs.
Revolutionary Technological Advances and Clinical Breakthroughs That Are Redefining Efficacy and Safety Standards in Lung Stenting Procedures
Revolutionary Technological Advances and Clinical Breakthroughs That Are Redefining Efficacy and Safety Standards in Lung Stenting Procedures
In recent years, the migration from conventional metallic frameworks to bioresorbable and drug-eluting constructs has redefined long-term patient outcomes and procedural safety. Innovations in magnesium-based alloys and PLLA-based resorbable scaffolds have shown promise in minimizing chronic inflammation and late-stage restenosis, while advanced polymer coatings enable controlled drug release that targets proliferative airway tissue. Concurrently, refinements in nitinol and stainless steel designs, paired with polycarbonate urethane components, have enhanced radial force profiles and fatigue resistance, elevating the performance benchmarks for stent durability.
Moreover, the integration of high-resolution bronchoscopy and intraoperative imaging modalities facilitates more precise placement, reducing peri-operative complications and the incidence of migration. Novel customization approaches, including patient-specific 3D printing and adaptive sizing algorithms, are fostering a new era of personalized interventional therapy. Collectively, these breakthroughs underscore a paradigm shift from one-size-fits-all devices toward customizable, biologically integrated stents that align closely with individual airway geometries and tissue healing responses.
Analyzing the Long-Term Effects of Recent United States Tariff Measures on the Supply Chain Dynamics and Cost Structures of Lung Stent Technologies
Analyzing the Long-Term Effects of Recent United States Tariff Measures on the Supply Chain Dynamics and Cost Structures of Lung Stent Technologies
At the outset of 2025, the United States introduced revised tariff schedules targeting select medical device imports, including critical components used in manufacturing lung stents. These measures aimed to reinforce domestic production capacity, yet they have also contributed to increased input costs for raw materials such as specialized alloys and polymer precursors. In response, manufacturers have re-evaluated supplier agreements, accelerating nearshore and onshore partnerships to mitigate exposure to elevated duty assessments and logistics complexities.
Consequently, procurement teams at health systems and device companies have experienced shifts in lead times and pricing volatility. Rather than relying solely on traditional cross-border sourcing strategies, many stakeholders are investing in dual-sourcing arrangements and regional distribution hubs to maintain supply continuity. Over time, these adjustments are expected to recalibrate cost structures, favor domestic manufacturing investment, and strengthen resilience against future policy fluctuations. Understanding these dynamics is essential for CFOs and supply chain executives who aim to anticipate budgetary impacts and uphold timely access to lifesaving airway therapies.
Unveiling Critical Patient, Product and Distribution Segmentation Patterns That Inform Strategic Positioning in the Lung Stents Market
Unveiling Critical Patient, Product and Distribution Segmentation Patterns That Inform Strategic Positioning in the Lung Stents Market
The lung stents domain encompasses a variety of product types, each tailored to specific clinical needs. Bioresorbable stents, available in magnesium-based and PLLA-based formulations, offer temporary scaffolding that gradually dissolves, reducing the need for secondary retrieval procedures. Drug-eluting stents deploy antiproliferative therapies directly at the site of implantation, while traditional metallic and polymer variants-comprising biodegradable and nondegradable polymers-remain foundational for diverse intervention strategies.
Clinical indications further segment the landscape, spanning malignant and benign tumor management, airway obstruction relief, malacia stabilization, and tracheobronchial stenosis correction. Tumor-focused applications demand precision drug delivery for palliative care, whereas malacia interventions prioritize dynamic support to counteract airway collapse. Material science divides offerings into nitinol superelastic frames, polymer-based constructs utilizing poly-L-lactic acid and polycarbonate urethane, and classic stainless steel designs valued for their established performance profile.
Patient demographics reveal distinct requirements across pediatric, adult, and geriatric cohorts, as younger patients may necessitate growth-accommodating technologies while older patients often present with comorbidities influencing device selection. Finally, the choice of distribution channel-from ambulatory surgical centers to outpatient clinics and hospital networks-affects procedural workflows, reimbursement considerations, and customer engagement models. Together, these segmentation insights guide stakeholders in tailoring offerings and go-to-market strategies to specific clinical and operational contexts.
This comprehensive research report categorizes the Lung Stents market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Product Type
- Indication
- Material
- Patient Age Group
- Distribution Channel
Exploring Regional Disparities and Growth Drivers Across North America, Europe Middle East Africa and Asia Pacific for Lung Stent Adoption
Exploring Regional Disparities and Growth Drivers Across North America, Europe Middle East Africa and Asia Pacific for Lung Stent Adoption
Across the Americas, robust reimbursement environments and established referral networks have enabled early adoption of advanced stent technologies, particularly in tertiary care centers and leading academic hospitals. In addition, proactive regulatory pathways have facilitated faster device approvals, resulting in a steady influx of next-generation materials and drug-eluting solutions. Meanwhile, emerging markets in Latin America are demonstrating rising procedural volumes as healthcare infrastructure expands and awareness of minimally invasive airway therapies grows.
Within Europe, Middle East and Africa, heterogeneous regulatory frameworks present both challenges and opportunities. Western European markets exhibit high demand for cutting-edge, personalized stenting solutions, supported by comprehensive health coverage schemes. In contrast, regions across the Middle East and Africa are prioritizing capacity building and training to widen access to core airway interventions, with emphasis on cost-effective stainless steel and basic polymer platforms.
The Asia Pacific region is witnessing substantial growth driven by large patient populations, increased diagnostic capabilities, and government initiatives to upgrade interventional pulmonology services. Local manufacturing capabilities are also maturing, leading to competitive regional players that challenge established global brands. This diverse regional landscape demands nuanced market strategies that account for reimbursement structures, healthcare delivery models, and local innovation ecosystems.
This comprehensive research report examines key regions that drive the evolution of the Lung Stents market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Profiling Leading Innovators and Strategic Collaborations Shaping Competitive Dynamics in the Global Lung Stent Industry
Profiling Leading Innovators and Strategic Collaborations Shaping Competitive Dynamics in the Global Lung Stent Industry
Key participants in the lung stent arena are advancing their product pipelines through targeted research and partnership agreements. Established medical technology firms are leveraging their global distribution networks to introduce proprietary bioresorbable and drug-eluting platforms, while also expanding clinical trial footprints to generate robust safety and efficacy datasets. Concurrently, specialized device startups are forging alliances with academic centers to accelerate proof-of-concept validation for next-generation materials and smart stent systems that can deliver diagnostic feedback.
Strategic collaborations between component suppliers, contract manufacturers, and interventional pulmonology experts are driving cost efficiencies and iterative design improvements. Joint ventures aimed at regional production hubs are gaining traction as companies seek to circumvent tariff constraints and reduce lead times. Moreover, cross-sector partnerships with digital health providers and imaging technology vendors are enabling integrated solutions that enhance procedural planning and post-implantation monitoring. These competitive dynamics highlight the multifaceted approaches firms are employing to secure market share and sustain innovation pipelines.
This comprehensive research report delivers an in-depth overview of the principal market players in the Lung Stents market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Boston Scientific Corporation
- BVM Medical Limited
- Cook Medical LLC
- Medtronic plc
- Meril Life Sciences Pvt. Ltd
- Merit Medical Systems, Inc.
- Novatech SA
- Stening SRL
- Taewoong Medical Co., Ltd
- W. L. Gore & Associates, Inc.
Implementing Data-Driven Strategic Initiatives and Operational Best Practices to Enhance Market Penetration and Patient Outcomes in Lung Stenting
Implementing Data-Driven Strategic Initiatives and Operational Best Practices to Enhance Market Penetration and Patient Outcomes in Lung Stenting
Organizations aiming to excel in the lung stent sector should prioritize the alignment of R&D investments with real-world clinical evidence. By systematically collecting and analyzing patient outcome data, firms can refine design parameters and substantiate differentiating claims in regulatory submissions. Adopting a modular product development strategy that accommodates customizable features for specific anatomical and disease states will further differentiate offerings and respond to evolving physician preferences.
Additionally, reinforcing supply chain resilience through diversified sourcing strategies and regional manufacturing alliances will mitigate the financial and operational impacts of tariff adjustments. Collaborating closely with reimbursement experts and key opinion leaders will also streamline market access and underscore the value proposition of premium stent technologies. On the commercial front, educational outreach programs that demonstrate procedural efficiencies and patient quality-of-life improvements will accelerate uptake across both high-volume centers and community-based care settings.
Detailing a Robust Mixed-Method Analytical Framework Incorporating Primary Expert Consultations and Comprehensive Secondary Data Synthesis
Detailing a Robust Mixed-Method Analytical Framework Incorporating Primary Expert Consultations and Comprehensive Secondary Data Synthesis
This report’s methodology integrates primary insights derived from in-depth interviews with interventional pulmonologists, device engineers, supply chain executives, and reimbursement specialists, ensuring a 360-degree perspective on innovation trends and adoption barriers. These qualitative engagements were complemented by secondary research encompassing regulatory filings, peer-reviewed literature, clinical trial registries, and device patent databases to validate emerging material science breakthroughs and procedural advancements.
Quantitative analysis involved mapping tariff schedules, clinical adoption rates, and regional procedural volumes to identify correlations between policy shifts and market dynamics. Through triangulation of these data points, the framework delivers a holistic understanding of cost-structure evolution, competitive positioning, and patient cohort segmentation. Strict quality control measures, including cross-validation against public health statistics and triangulation across multiple data sources, underpin the rigor and reliability of the findings presented herein.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Lung Stents market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Lung Stents Market, by Product Type
- Lung Stents Market, by Indication
- Lung Stents Market, by Material
- Lung Stents Market, by Patient Age Group
- Lung Stents Market, by Distribution Channel
- Lung Stents Market, by Region
- Lung Stents Market, by Group
- Lung Stents Market, by Country
- United States Lung Stents Market
- China Lung Stents Market
- Competitive Landscape
- List of Figures [Total: 17]
- List of Tables [Total: 1590 ]
Summarizing Strategic Implications and Future Prospects to Empower Stakeholders and Drive Sustainable Innovation in Lung Stent Technologies
Summarizing Strategic Implications and Future Prospects to Empower Stakeholders and Drive Sustainable Innovation in Lung Stent Technologies
The convergence of material innovation, regulatory evolution, and shifting policy landscapes signals a pivotal moment for lung stent developers and healthcare providers alike. Stakeholders that cultivate agility in their product roadmaps, supply chain models, and market access strategies will be best positioned to capitalize on emerging clinical needs and reimbursement paradigms. Emphasizing collaborative partnerships with research institutions and digital health innovators will further accelerate the translation of laboratory breakthroughs into commercial realities.
Looking ahead, the integration of smart sensing functionalities, adaptive drug delivery systems, and patient-specific design methodologies is poised to propel the next wave of lung stent advancements. As the market continues to mature, sustained investment in clinical evidence generation, regulatory alignment, and stakeholder education will differentiate leaders from laggards. This executive summary underscores the strategic imperatives that must guide organizational decision-making to ensure both patient-centric outcomes and long-term commercial success in the evolving lung stents landscape.
Unlock Comprehensive Lung Stent Market Intelligence and Strategic Guidance Through a Tailored Consultation with Associate Director of Sales and Marketing
To unlock a comprehensive understanding of how evolving clinical practices and strategic market forces will shape the future of lung stent adoption, reach out for a personalized consultation with Associate Director, Sales & Marketing, Ketan Rohom. During this consultation, you will explore tailored insights on segment performance, regulatory trajectories, and competitive readiness to inform high-impact decision making. Engage directly with our expert team to outline bespoke solutions for your organization’s unique challenges and growth objectives, from building resilient supply chains in the wake of new tariff regimes to accelerating innovation in bioresorbable and drug-eluting platforms. This call will equip you with the clarity and foresight needed to seize emerging opportunities, optimize resource allocation, and establish enduring leadership in the lung stent arena. Contact Ketan Rohom today to secure your place at the forefront of strategic market intelligence and ensure your stakeholders are armed with actionable, up-to-date guidance derived from rigorous primary research and in-depth analysis.

- How big is the Lung Stents Market?
- What is the Lung Stents Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




