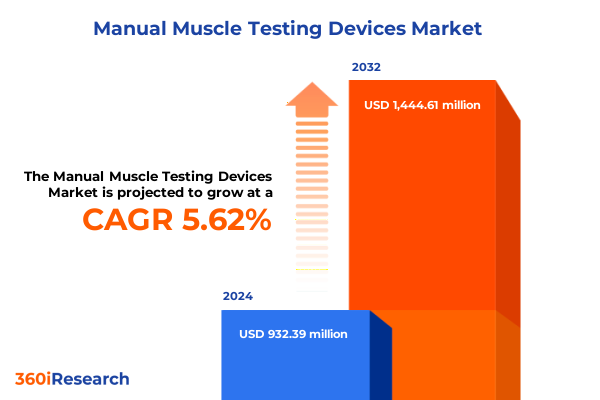
The Manual Muscle Testing Devices Market size was estimated at USD 982.97 million in 2025 and expected to reach USD 1,037.52 million in 2026, at a CAGR of 5.65% to reach USD 1,444.61 million by 2032.
Establishing the Role of Manual Muscle Testing Devices in Enhancing Rehabilitation Outcomes and Driving Innovation in Diagnostic and Performance Evaluation
Manual muscle testing devices have become indispensable in modern rehabilitation, diagnostics, and performance evaluation, forming the cornerstone of strength assessment across diverse healthcare settings. Over recent years, advances in sensor technologies and digital integration have elevated these instruments from simple manual tools to sophisticated systems capable of capturing precise force measurements in real time. Consequently, clinicians can now track patient progress with enhanced accuracy, while researchers leverage rich data streams to uncover new insights into muscular function and recovery patterns. This evolution underscores the growing importance of these devices in optimizing therapeutic interventions and guiding evidence-based decision making.
This executive summary provides a concise yet comprehensive overview of the critical factors driving the manual muscle testing devices sector today. It highlights key technological shifts, regulatory developments, and market forces shaping strategic decision making. Additionally, it explores the implications of new tariffs in the United States, segmentation and regional dynamics, leading competitor strategies, and actionable recommendations for industry stakeholders. By synthesizing these elements, readers are equipped with the context needed to navigate the complex landscape and capitalize on emerging growth opportunities without relying on numerical forecasts or market sizing data.
Unveiling the Transformative Shifts Reshaping Manual Muscle Testing Through Technological Innovation, Digital Integration, and Evolving Clinical Practices
The landscape of manual muscle testing devices is undergoing a profound transformation driven by breakthroughs in digital health, connectivity, and patient-centric design. Historically reliant on mechanical gauges and manual recording, the field has witnessed a surge in electronic dynamometers that integrate load cell and strain gauge sensors to deliver instantaneous feedback. Coupled with cloud-based platforms and secure data management, these devices now facilitate remote monitoring and tele-rehabilitation, enabling clinicians to extend care beyond traditional settings. As a result, new paradigms in chronic disease management and performance coaching have emerged, reshaping how strength assessments inform treatment and training regimens.
In parallel, regulatory frameworks and quality standards have evolved to address safety and interoperability, prompting manufacturers to adopt modular designs and standardized data protocols. This shift has accelerated collaborations between device makers and software developers, yielding intuitive interfaces and advanced analytics tools that translate raw force readings into actionable clinical insights. Consequently, stakeholders across hospitals, rehabilitation centers, and sports clinics can harness a more holistic view of muscular health, reinforcing the transition from episodic evaluations to continuous performance optimization.
Analyzing How 2025 United States Tariff Changes on Manual Muscle Testing Devices Are Affecting Supply Chains, Costs, and Market Dynamics
In 2025, the United States implemented revised tariffs on imported components and finished units within the manual muscle testing device category, marking a significant inflection point for supply chain economics. These measures have led to recalibrated procurement strategies as manufacturers evaluate alternative sourcing options to mitigate elevated import duties. In response, some firms have accelerated localization of critical components such as hydraulic actuators and electronic sensors, while others have renegotiated long-term contracts to absorb incremental cost pressures without passing them fully to end users.
Over time, distributors and clinical end users have experienced shifts in pricing and inventory turnover, prompting greater emphasis on cost-effective calibrations and modular service models. Although near-term concerns centered on transactional expenses, the cumulative effect has spurred investments in domestic manufacturing infrastructure and vertical integration. As a result, stakeholders across home care markets, hospitals, and sports clinics are adapting to a landscape where tariff resilience and supply chain agility have become paramount to sustaining competitive positioning.
Discovering Strategic Insights Derived from Product Type, End User Profiles, Technological Variants, and Distribution Pathways in Manual Muscle Testing Devices
A nuanced understanding of segmentation across product typologies, end-user categories, technology variants, and distribution pathways reveals strategic levers for differentiation. Within the product portfolio, electronic dynamometers lead in precision applications, complemented by portable handheld dynamometers for point-of-care flexibility, while isokinetic dynamometers serve specialized rehabilitation and athletic training programs, and mechanical dynamometers maintain relevance where simplicity and cost-containment are paramount. Each product class addresses distinct clinical needs, from rigorous research protocols to mobile wellness assessments.
Exploring end-user segmentation further refines market positioning, as settings ranging from home care environments to hospitals, rehabilitation centers, research institutes, and sports clinics exhibit divergent adoption drivers. Home care scenarios value user-friendly interfaces and remote data transfer, whereas hospitals and research institutes demand integrated analytics and compliance with stringent certification standards. Technology choices further differentiate solutions, with electromagnetic systems offering smooth variable resistance, hydraulic models providing robust durability, load cell and strain gauge architectures ensuring precise measurement, and pneumatic designs balancing responsiveness with safety.
Distribution strategies also carry significant weight, as direct sales channels foster consultative relationships and customized deployments, distributors expand market reach through localized networks, online sales platforms cater to digital-first procurement trends, and retail channels enhance visibility among non-traditional users. By weaving these segmentation threads together, stakeholders can identify unmet needs and tailor offerings to achieve optimal market resonance.
This comprehensive research report categorizes the Manual Muscle Testing Devices market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Product Type
- Technology
- End User
- Distribution Channel
Mapping Regional Performance and Growth Drivers across the Americas, Europe Middle East Africa, and Asia Pacific Markets for Manual Muscle Testing Equipment
Regional analysis underscores distinct growth trajectories influenced by demographic, regulatory, and economic factors. In the Americas, an aging population combined with advanced reimbursement frameworks has solidified demand for muscle strength assessment tools across rehabilitation clinics and ambulatory care settings. The presence of well-established sports medicine networks and academic research hubs further amplifies adoption, reinforcing the region’s leadership in innovation.
Across Europe, Middle East, and Africa, harmonized regulatory standards such as Medical Device Regulation (MDR) in Europe and increasing healthcare investment in Gulf Cooperation Council countries are unlocking new opportunities. Clinical decision makers in these markets increasingly favor integrated digital platforms that streamline data workflows and comply with evolving safety mandates, while ongoing infrastructure upgrades in emerging African markets are expanding the addressable base for portable testing solutions.
In the Asia-Pacific region, rapid urbanization, rising healthcare expenditure, and an expanding middle class are driving broader access to rehabilitation and sports performance services. Local manufacturing initiatives in East Asia and government health campaigns in South Asian economies incentivize tele-health and home-based diagnostics, positioning the region as a hotbed for novel device form factors and digital service models to proliferate.
This comprehensive research report examines key regions that drive the evolution of the Manual Muscle Testing Devices market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Highlighting Competitive Strategies, Innovation Portfolios, and Strategic Partnerships of Leading Players in the Manual Muscle Testing Devices Landscape
Leading players in the manual muscle testing space are deploying diverse strategies to reinforce their market presence and fuel long-term growth. Technology pioneers have expanded their solution suites by integrating artificial intelligence-driven analytics that transform static force readings into predictive performance metrics. These enhancements enable clinicians and trainers to anticipate muscular imbalances and optimize intervention protocols.
Strategic partnerships and targeted acquisitions have also been instrumental, as firms align with specialized sensor manufacturers and software developers to accelerate time to market for next-generation devices. Companies emphasizing modularity and service-based revenue models are cultivating stronger ties with hospitals and rehabilitation centers by offering ongoing calibration, training, and software updates as part of subscription-style engagements.
In parallel, a subset of innovators has deepened focus on ergonomic design and portability, collaborating with sports clinics and research institutes to co-create custom fixture systems and mobile application interfaces. By combining product ingenuity with comprehensive support ecosystems, these leaders are establishing new customer expectations around device performance, user experience, and total cost of ownership.
This comprehensive research report delivers an in-depth overview of the principal market players in the Manual Muscle Testing Devices market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- AMETEK, Inc.
- Biometrics Ltd.
- Delsys, Inc.
- EvalTech, Inc.
- Fabrication Enterprises, Inc.
- Hoggan Health Industries, Inc.
- JTECH Medical, Inc.
- Lafayette Instrument Company
- Patterson Companies, Inc.
- RTI Electronics, Inc.
Empowering Industry Leaders with Tactical Recommendations for Navigating Regulatory Complexities, Advancing Technology Adoption, and Expanding Market Reach
Industry leaders aiming to secure a competitive advantage should prioritize investments in digital ecosystems that seamlessly connect devices, analytics platforms, and electronic health records. By embedding real-time data capture and automated reporting features, manufacturers can enhance clinical workflows, reduce manual errors, and strengthen value propositions for end users. Moreover, designing open application programming interfaces (APIs) will facilitate interoperability with tele-rehabilitation solutions and third-party software, expanding market reach and fostering collaborative innovation.
To address tariff-driven supply chain complexities, stakeholders are advised to diversify supplier networks and develop localized production capabilities for key components. Establishing strategic alliances with regional partners not only mitigates duty exposure but also accelerates response times for calibration services and equipment upgrades. Additionally, offering tiered service packages that align with the needs of home care providers, hospitals, and research institutes can unlock new revenue streams and deepen customer engagement.
Finally, aligning product roadmaps with emerging regulatory guidelines and end-user preferences-such as customizable resistance protocols and patient-friendly interfaces-will position companies to capture early mindshare in adjacent markets. Emphasizing continuous education and certification programs for clinicians and trainers further amplifies brand credibility and drives sustained technology adoption.
Elucidating the Comprehensive Research Methodology Combining Primary Stakeholder Consultations, Secondary Data Mining, and Analytical Frameworks
This research combines rigorous primary and secondary methodologies to ensure comprehensive coverage and robust validation of insights. Primary inputs were collected through in-depth interviews with clinical experts, rehabilitation specialists, device engineers, and procurement decision makers across diverse care settings. These conversations yielded firsthand perspectives on product performance criteria, technology adoption hurdles, and regulatory compliance challenges.
Secondary research entailed systematic reviews of peer-reviewed journals, academic conference proceedings, patent databases, and regulatory agency publications. Data triangulation was achieved by cross-referencing device specification sheets, clinical trial outcomes, and manufacturer white papers. Historical trend analysis was supported by examining industry association reports and electronic health record integration studies.
Analytical frameworks including SWOT analysis, Porter’s Five Forces, and scenario planning were applied to interpret qualitative and quantitative data. Key themes were distilled through iterative workshops with technical advisors and validation rounds with end-user focus groups. This multi-layered approach ensures that the findings accurately reflect current market realities while anticipating future inflection points in the manual muscle testing devices sector.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Manual Muscle Testing Devices market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Manual Muscle Testing Devices Market, by Product Type
- Manual Muscle Testing Devices Market, by Technology
- Manual Muscle Testing Devices Market, by End User
- Manual Muscle Testing Devices Market, by Distribution Channel
- Manual Muscle Testing Devices Market, by Region
- Manual Muscle Testing Devices Market, by Group
- Manual Muscle Testing Devices Market, by Country
- United States Manual Muscle Testing Devices Market
- China Manual Muscle Testing Devices Market
- Competitive Landscape
- List of Figures [Total: 16]
- List of Tables [Total: 795 ]
Synthesizing Key Insights and Implications to Guide Strategic Decisions and Foster Innovation in the Manual Muscle Testing Devices Sector
The insights presented in this summary underscore the multifaceted evolution of manual muscle testing devices, driven by technological innovation, regulatory shifts, and complex supply chain dynamics. Segmentation analysis reveals that targeted product development, aligned with specific end-user requirements and distribution models, creates differentiated value propositions. Regional perspectives highlight how demographic trends, healthcare policies, and economic priorities shape adoption patterns across the Americas, EMEA, and Asia-Pacific.
Furthermore, the 2025 tariff adjustments in the United States have catalyzed supply chain optimization and localized manufacturing, emphasizing the strategic imperative of resilience. The competitive landscape is defined by companies that excel in integrating digital ecosystems, forging strategic partnerships, and delivering comprehensive service offerings. Looking ahead, organizations that proactively embrace open architectures, regulatory preparedness, and user-centric design will be best positioned to navigate uncertainties and drive sustained growth.
Unlock In-Depth Intelligence and Accelerate Strategic Growth by Partnering with Ketan Rohom to Gain Access to the Manual Muscle Testing Devices Research Report
By opting to partner with Ketan Rohom, Associate Director for Sales & Marketing, your organization will secure unparalleled access to the full suite of findings and analyses on manual muscle testing devices. This comprehensive report distills the most critical market dynamics, emerging opportunities, and strategic imperatives shaping the sector. Ketan brings deep expertise in translating complex research into actionable insights and is ready to guide you through the nuances of device innovation, regulatory considerations, and evolving end-user demands. Engaging directly with his team ensures you gain not only the data but also the contextual understanding necessary to make fast, informed decisions that drive competitive advantage. Reach out to Ketan today to arrange a personalized briefing, explore tailored licensing options, and accelerate your path to market leadership by leveraging the definitive resource on manual muscle testing devices

- How big is the Manual Muscle Testing Devices Market?
- What is the Manual Muscle Testing Devices Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




