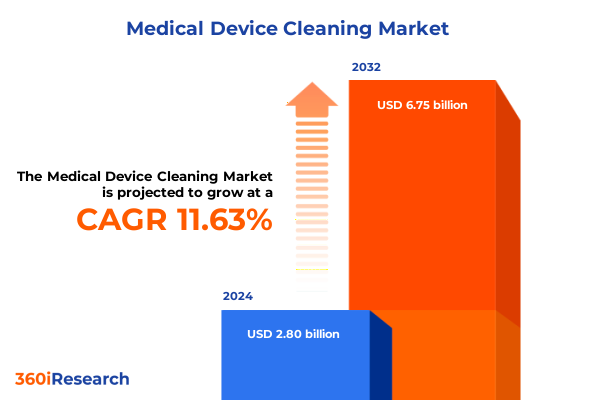
The Medical Device Cleaning Market size was estimated at USD 22.44 billion in 2025 and expected to reach USD 23.59 billion in 2026, at a CAGR of 6.74% to reach USD 35.43 billion by 2032.
Unveiling the Critical Role of Medical Device Cleaning in Safeguarding Patient Outcomes, Ensuring Regulatory Compliance, and Enhancing Operational Efficiency
Comprehensive cleaning and sterilization of medical devices sits at the heart of patient safety protocols, serving as a fundamental barrier against healthcare-associated infections that pose severe risks to vulnerable populations. In the United States alone, nearly one in 25 hospital patients develops a healthcare-associated infection annually, with more than 680,000 cases leading to approximately 72,000 deaths, highlighting the life-or-death importance of meticulous reprocessing practices for surgical instruments, endoscopes, and other critical equipment. The Centers for Disease Control and Prevention has documented meaningful declines in central line–associated bloodstream infections and catheter-associated urinary tract infections, pointing to the tangible impact of robust cleaning, disinfection, and sterilization measures when supported by standardized protocols and centralized sterile processing departments.
Regulatory frameworks such as ISO 17664 and the U.S. Food and Drug Administration’s endorsement of centralized cleaning operations reinforce the necessity of validated cleaning processes. These guidelines stipulate precise detergent specifications, contact times, and traceability requirements that ensure every high-risk reusable device is consistently free of organic residues and biofilms prior to disinfection or sterilization. As healthcare delivery evolves toward growing volumes of minimally invasive procedures and outpatient surgeries, the foundational role of medical device cleaning as both a clinical safeguard and an operational imperative continues to intensify, demanding advanced technologies and rigorous compliance across every care setting.
The Rise of Automation, AI, IoT Integration, and Sustainable Practices Redefining Protocols, Regulatory Compliance, and Efficiency in Medical Device Cleaning
Across healthcare facilities worldwide, medical device cleaning protocols are experiencing a technological renaissance driven by automation, artificial intelligence, and digital connectivity. Automated washer-disinfectors equipped with precision sensors now dominate high-volume hospital environments by minimizing human error and delivering repeatable, validated cleaning cycles that support audit readiness and regulatory adherence. Concurrently, the integration of IoT platforms allows real-time monitoring of key parameters-pH, temperature, and cycle duration-facilitating immediate corrective actions when cleaning efficacy deviates from established thresholds.
The industry’s pivot toward sustainability is equally transformative, as manufacturers innovate biodegradable, non-toxic detergents and low-foam, pH-neutral formulations that balance environmental stewardship with rigorous bioburden removal. These eco-friendly agents align with global directives to reduce chemical waste in healthcare wastewater systems and improve worker safety without compromising instrument integrity. At the same time, anticipated updates to the FDA’s Quality Management System Regulation, set to harmonize with ISO 13485 and the EU Medical Device Regulation, underscore an impending era of globally aligned cleaning standards that will reward providers and suppliers capable of delivering transparent, evidence-based reprocessing documentation and fully integrated cleaning solutions.
Assessing the Cumulative Impact of United States' 2025 Tariffs on Medical Device Cleaning Supplies, Equipment, and Supply Chain Resilience
The implementation of Section 301 tariff adjustments on January 1, 2025 has ushered in significant cost pressures across the medical device cleaning supply chain. Tariffs on rubber medical and surgical gloves surged to 50%, while duties on disposable textile facemasks increased to 25%, amplifying consumable expenses for high-volume items critical to daily reprocessing workflows. Simultaneously, duties rose to 100% on syringes and needles and 50% on semiconductor components essential to automated cleaning system electronics. Additional levies on steel and aluminum derivatives took effect on March 12, 2025 at a rate of 25%, impacting washer-disinfector construction and repair parts imported from key manufacturing hubs.
Given that medical supply expenditures represent over 10% of the average U.S. hospital’s budget, adding an estimated $6.6 billion in tariff-related costs year over year, stakeholders must adapt purchasing strategies and explore domestic sourcing alternatives to preserve both quality and financial sustainability. While pharmaceutical imports remain largely exempt, the broad applicability of these measures to surgical instruments, reprocessor components, and cleaning chemistries underscores the urgency for healthcare systems to advocate for targeted exemptions and invest in vertically integrated supply models to mitigate future trade disruptions.
In-Depth Analysis of Market Segmentation Reveals Nuanced Cleaning Methods, Product Types, End Users, and Distribution Channel Dynamics
Differentiation by cleaning method reveals distinct operational trade-offs: automated cleaning systems are increasingly favored in centralized sterile processing departments for their ability to deliver validated, reproducible decontamination cycles, while manual cleaning techniques remain indispensable at point-of-use for devices requiring immediate pre-cleaning to prevent organic matter from drying onto instruments. This dual pathway ensures that both intricate endoscopes and high-throughput surgical trays can undergo optimal pre-conditioning prior to final disinfection or sterilization.
Examining product types highlights varied chemistries and technologies. Within consumables, alkaline detergents and enzymatic formulations have gained prominence for their efficacy against proteinaceous soils, while neutral pH solutions protect delicate device materials. Equipment segmentation spotlights endoscope reprocessors as critical assets in gastroenterology suites, ultrasonic cleaners as versatile solutions for dental and research labs, and washer-disinfectors as the backbone of hospital CSSDs. Services underpin these offerings, with maintenance contracts ensuring uninterrupted operation, training modules equipping technicians with IFU-driven protocols, and validation services certifying compliance with regulatory benchmarks.
End user insights demonstrate that private hospitals leverage advanced automated solutions to address large procedural volumes, whereas public hospitals balance budget constraints with centralized reprocessing investments. Ambulatory surgical centers, both hospital-owned and independent, increasingly adopt compact washer-disinfectors to support outpatient workflows. Dental clinics prioritize compact ultrasonic systems for instrument hygiene, while pharmaceutical companies and research laboratories demand bespoke cleaning protocols and devices that meet stringent particulate and bioburden thresholds. Distribution dynamics reflect a direct sales emphasis for major healthcare networks, reliance on regional distributors by mid-sized providers, and an acceleration of online procurement channels for consumable replenishment.
This comprehensive research report categorizes the Medical Device Cleaning market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Cleaning Method
- Product Type
- End User
- Distribution Channel
Regional Spotlight on Medical Device Cleaning Trends Illuminates Distinct Drivers Across the Americas, EMEA, and Asia-Pacific Markets
In North America, stringent FDA and CDC directives have catalyzed rapid adoption of high-efficiency automated cleaning systems, complemented by investments in local manufacturing to insulate supply chains from tariff shocks and global logistics disruptions. The region’s robust reimbursement frameworks and infection prevention mandates propel hospitals and outpatient facilities to pursue validated cleaning workflows, driving R&D partnerships and capital deployment in state-of-the-art sterile processing technologies.
Within Europe, Middle East, and Africa, harmonization under the EU Medical Device Regulation and ISO standards fosters cross-border compatibility of cleaning chemistries and equipment. Healthcare providers in Western Europe emphasize eco-friendly solutions in alignment with stringent environmental directives, while regulatory acceleration in emerging Middle Eastern and African markets spurs demand for modular cleaning units and turnkey services to elevate infection control capabilities.
Asia-Pacific stands as the fastest growing region, fueled by escalating healthcare investments, burgeoning outpatient procedure volumes, and a thriving network of third-party sterile reprocessing organizations. Countries such as China and India lead supply-side innovation with domestic equipment manufacturing, while Australia and Japan drive early adoption of IoT-enabled monitoring systems. The convergence of government funding, infrastructure expansion, and heightened public awareness around infection prevention positions Asia-Pacific as a pivotal growth arena for advanced medical device cleaning solutions.
This comprehensive research report examines key regions that drive the evolution of the Medical Device Cleaning market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Strategic Insights into Leading Medical Device Cleaning Companies Spotlight Innovation, Mergers, and Competitive Dynamics Shaping the Industry
Leading enterprises are advancing the frontier of medical device cleaning through targeted R&D, strategic partnerships, and acquisitions that broaden their solution portfolios. STERIS plc has solidified its market leadership by integrating surgical instrumentation assets from Becton, Dickinson, and Company and executing the landmark merger with Cantel Medical, expanding its footprint in endoscopy and dental sterilization offerings. Continued investment in enzyme-based chemistries and digital process controls further reinforces STERIS’s dominance in high-risk device reprocessing.
Getinge Group channels its innovation agenda into next-generation steam sterilizers and automated washing systems, exemplified by the launch of the Solsus 66 steam sterilizer optimized for enhanced throughput and reliability in high-volume hospital settings. Ecolab, with its suite of eco-minded cleaning solutions, pursues synergistic collaborations that meld sustainability with rigorous microbial efficacy, while Metrex Research focuses on antimicrobial surface treatments and point-of-use interventions tailored for perioperative use. These market players leverage digital service platforms, regional distribution alliances, and compliance-certified validation services to navigate evolving regulatory landscapes and customer expectations.
Smaller innovators, including Rhuof Corporation and Oro Clean Chemie AG, bring niche enzymatic and acid-based formulations that target specific bioburden challenges, attracting partnerships with tertiary care centers and specialty clinics seeking differentiated performance against resilient pathogens. Collectively, these companies shape a competitive ecosystem marked by convergence of chemistry expertise, automation acumen, and service orchestration.
This comprehensive research report delivers an in-depth overview of the principal market players in the Medical Device Cleaning market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- 3M Company
- ASP Global Manufacturing GmbH
- BODE Chemie GmbH by HARTMANN GROUP
- Ecolab Inc.
- Fortive Corporation
- GAMA Healthcare Ltd.
- Getinge AB
- Medline Industries, Inc.
- Metrex Research LLC
- Micro-Scientific, LLC
- MMM Münchener Medizin Mechanik GmbH by WSP Global Inc.
- Olympus Corporation
- Oro Clean Chemie AG
- Ruhof Corporation
- Scican Ltd. by COLTENE GROUP
- Soluscope SAS
- Steelco S.p.A.
- Steris Corporation
- Tristel Solutions Ltd.
- Tuttnauer B.V.
- Wassenburg Medical B.V. by HOYA Corporation
Actionable Strategies for Industry Leaders to Drive Innovation, Build Resilience, and Navigate Regulatory and Supply Chain Challenges
Healthcare leaders should accelerate integration of automated cleaning systems and robotics in centralized sterile processing functions to improve consistency, reduce labor dependencies, and enhance traceability. By partnering with technology providers to co-develop IoT-enabled monitoring platforms, organizations can secure real-time visibility of reprocessing metrics, enabling proactive quality control and audit readiness.
Procurement strategies must pivot toward diversified sourcing models that balance tariff-induced cost pressures with supply chain resilience. Engaging domestic manufacturers for critical consumables and precursor materials while advocating for medical device exemptions under Section 301 tariffs can buffer financial headwinds and safeguard continuous access to essential supplies.
Infection prevention teams should collaborate with clinical engineers and infection control professionals to standardize manufacturer IFUs, ensuring they are concise, evidence-based, and seamlessly integrated into electronic tracking systems. Clear, accessible instructions reduce citation risks and empower frontline technicians to execute validated cleaning cycles universally.
Finally, embedding sustainability criteria into cleaning program evaluations-prioritizing biodegradable chemistries and energy-efficient equipment-aligns with institutional environmental goals while bolstering organizational reputation among patients and payors seeking green healthcare solutions.
Comprehensive Overview of Research Methodology Demonstrates Rigorous Data Collection, Analysis Frameworks, and Validation Processes
This research harnesses a hybrid methodology, blending primary interviews with sterile processing experts, infection preventionists, and procurement executives alongside secondary data drawn from regulatory agencies, industry association reports, and leading consultancy briefs. Insights from CDC surveillance of healthcare-associated infections and USTR documentation of Section 301 tariff actions inform validation of cost and compliance variables. White & Case’s analysis of tariff schedules and FDA guidance on cleaning validation under ISO 17664 provided a legal and procedural framework for assessing trade and regulatory impacts.
Segment-specific data on cleaning methods, product categories, end users, and distribution channels were triangulated through vendor disclosures, equipment shipment data, and practitioner surveys to ensure representativeness across hospital, outpatient, and laboratory settings. Regional analyses incorporate macroeconomic indicators, healthcare expenditure profiles, and in-country regulatory alignment, enabling a comparative lens on adoption drivers and barriers. Rigorous cross-referencing with expert panel reviews and peer benchmarking underpins the credibility of findings and supports actionable takeaways for stakeholders seeking to optimize device reprocessing strategies and mitigate emerging trade risks.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Medical Device Cleaning market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Medical Device Cleaning Market, by Cleaning Method
- Medical Device Cleaning Market, by Product Type
- Medical Device Cleaning Market, by End User
- Medical Device Cleaning Market, by Distribution Channel
- Medical Device Cleaning Market, by Region
- Medical Device Cleaning Market, by Group
- Medical Device Cleaning Market, by Country
- United States Medical Device Cleaning Market
- China Medical Device Cleaning Market
- Competitive Landscape
- List of Figures [Total: 16]
- List of Tables [Total: 1590 ]
Concluding Perspectives Highlight Imperatives for Enhanced Cleaning Practices, Technological Adoption, and Collaborative Industry Advancement
The collective insights underscore that meticulous device cleaning stands as a foundational pillar in patient safety and operational efficiency, demanding continuous evolution in technology, protocols, and policy advocacy. Automation and digital integration have emerged as critical levers to standardize outcomes and support compliance across complex care environments. Simultaneously, sustainability imperatives and tariff disclosures highlight the intersection of environmental responsibility and supply chain stewardship in healthcare decision-making.
Segmentation nuances-spanning cleaning methods, product typologies, end user profiles, and channel strategies-reveal a dynamic landscape where tailored solutions resonate with distinct clinical and economic needs. Regional disparities further point to evolving regulatory harmonization and infrastructure maturation that will define next-wave adoption trajectories globally.
As market leaders navigate these currents, fostering cross-industry collaboration, investing in resilient sourcing models, and embedding evidence-based IFUs into electronic workflows will prove indispensable. The convergence of scientific rigor, technological innovation, and strategic partnerships forms the blueprint for advancing medical device cleaning excellence, ultimately safeguarding patient health and sustaining system-wide value.
Empower Your Decision-Making with Expert Medical Device Cleaning Insights—Connect with Ketan Rohom to Access the Complete Market Research Report
Elevate your strategic decisions with the full depth of our medical device cleaning market research report. To gain unrestricted access to detailed analyses, segmentation breakdowns, regional assessments, and company insights that drive competitive advantage, connect directly with Ketan Rohom, Associate Director of Sales & Marketing, today. Unlock the comprehensive knowledge needed to optimize your operations, navigate regulatory shifts, and capitalize on emerging opportunities in this critical sector.

- How big is the Medical Device Cleaning Market?
- What is the Medical Device Cleaning Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




