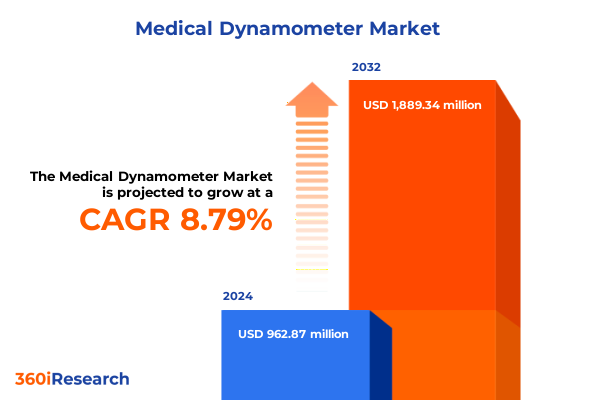
The Medical Dynamometer Market size was estimated at USD 1.02 billion in 2025 and expected to reach USD 1.10 billion in 2026, at a CAGR of 9.06% to reach USD 1.88 billion by 2032.
Comprehensive Introduction to the Medical Dynamometer Domain Highlighting Purpose, Scope, and Strategic Importance for Precision Healthcare Assessments
The medical dynamometer is an indispensable instrument in modern healthcare, providing precise measurements of muscular force that inform diagnoses, treatment plans and rehabilitation protocols. By translating biomechanical activity into quantifiable metrics, these devices enable clinicians to monitor patient progress objectively, fostering evidence-based decision-making across a spectrum of medical specialties. As healthcare systems increasingly prioritize patient outcomes and cost efficiencies, the significance of rigorous force assessment continues to grow.
Emerging sensor technologies and refined mechanical designs have propelled dynamometer capabilities, expanding usage beyond traditional physical therapy and sports medicine into geriatrics, research and orthopedics. Simultaneously, the shift toward personalized medicine has elevated the need for high-fidelity data, driving greater demand for devices that seamlessly integrate into electronic health records and telehealth platforms. Consequently, understanding the evolving landscape of medical dynamometers is critical for manufacturers, clinicians and investors aiming to align with industry trajectories.
This executive summary sets the stage for a strategic exploration of the market, delineating key industry dynamics, segmentation dimensions and external factors shaping adoption. It outlines the scope and methodology underpinning the underlying research, while highlighting the implications of technological advancements, regulatory shifts and global trade policies. The objective is to furnish decision-makers with a coherent, actionable synopsis that supports informed strategy development and sustainable competitive positioning.
Identifying Key Technological Advancements and Market Drivers Revolutionizing Medical Dynamometer Solutions for Enhanced Patient Outcomes and Efficiency
In recent years, breakthroughs in sensor miniaturization and digital signal processing have redefined the capabilities of medical dynamometers. High-precision load cells and strain gauge sensors now deliver unprecedented accuracy, while advanced pneumatic and hydraulic designs enable versatile force ranges tailored to diverse clinical needs. These technological strides have catalyzed the emergence of portable and table-top devices, bridging the gap between specialized laboratory equipment and point-of-care solutions.
The convergence of connectivity and analytics has further transformed market expectations. Ethernet-enabled and USB-compatible instruments now coexist with Bluetooth and Wi-Fi variants, facilitating real-time data transfer to electronic health record systems and remote monitoring platforms. This seamless integration boosts clinical efficiency by automating data capture, reducing transcription errors and supporting telemedicine initiatives. As a result, stakeholders are navigating a landscape where interoperability and cybersecurity are as pivotal as device performance.
Moreover, the industry is witnessing a shift toward modular and software-driven architectures. Open-platform frameworks allow third-party application integration, empowering researchers and clinicians to develop bespoke assessment protocols. Cloud-based analytics and AI-enhanced algorithms are unlocking predictive insights into muscle function and rehabilitation outcomes. Together, these transformative shifts are redefining the value proposition of medical dynamometers, positioning them as essential components in the broader ecosystem of connected healthcare solutions.
Analyzing the Cumulative Influence of 2025 United States Tariff Policies on Medical Dynamometer Supply Chains, Manufacturing Costs and Market Accessibility
The enactment of new tariff measures in 2025 has exerted significant pressure on the medical dynamometer supply chain. Imported steel, precision load cells and sensor components originating from global manufacturing hubs have become subject to heightened duties, translating into incremental material costs for original equipment manufacturers. In turn, this has prompted strategic reassessments of procurement policies and cost mitigation tactics aimed at preserving device affordability for healthcare providers.
In response, several industry players have accelerated localization initiatives, investing in domestic production of critical subassemblies and forging alliances with regional suppliers. These collaborations are designed to reduce exposure to fluctuating tariff schedules, stabilize lead times and cultivate resilience against further trade policy revisions. Furthermore, manufacturers are exploring vertically integrated models, acquiring specialized component makers to secure a steady flow of essential parts and insulate their operations from external volatility.
Simultaneously, the elevated cost environment has spurred innovation in material engineering, with designers experimenting with alternative alloys and composite structures that retain performance standards while mitigating tariff impacts. Regulatory incentives and government grants targeting reshoring efforts have reinforced these trends, enabling enterprises to offset capital expenditures associated with retooling and certification. Collectively, these shifts underscore a dynamic era in which strategic agility and supply-chain diversification are imperative for sustained competitiveness.
Uncovering Critical Segmentation Insights Illuminating Market Trends Across Technologies, Product Categories, Connectivity, Applications and End User Channels
An essential facet of market analysis is the evaluation of underlying technological frameworks that dictate device performance and application. Hydraulic dynamometers offer robust force measurement across wide ranges, while load cell variants deliver highly accurate digital outputs ideal for research and clinical diagnostics. Pneumatic configurations appeal to environments requiring clean, oil-free operation, whereas strain gauge models excel in compact, handheld form factors that benefit bedside assessments.
Product categorization further illuminates usage patterns and strategic positioning. Analog instruments, distinguished by dial-type displays or spring-based mechanisms, maintain prominence in resource-constrained settings due to their simplicity and cost-effectiveness. Conversely, digital offerings-spanning portable handheld units to versatile table-top systems-cater to advanced clinical workflows by providing data logging, trend analysis and seamless software interoperability through modular firmware enhancements.
Connectivity profiles reveal an ongoing transition from wired configurations to wireless ecosystems. Ethernet and USB interfaces continue to underpin legacy installations, offering reliable data transmission in controlled environments. However, Bluetooth-enabled devices and Wi-Fi-connected platforms are accelerating adoption in decentralized care models by enabling remote calibration, automated updates and integration with telehealth networks.
Examining application domains uncovers differentiated demand vectors. Geriatric assessments and orthopedic evaluations rely on consistent, reproducible metrics, while research laboratories exploit high-precision instruments to advance biomechanical studies. Physical therapy settings, both inpatient and outpatient, leverage dynamometers for patient monitoring and adaptive rehabilitation protocols. In sports medicine, preventive care specialists and rehabilitation teams apply dynamometric readings to optimize athlete performance and expedite recovery timelines.
A nuanced understanding of end-user channels informs distribution strategies across clinics, hospitals, research labs and sports rehabilitation centers. Outpatient clinics and specialized facilities prioritize portability and ease of use, whereas private and public hospital systems value integration with broader diagnostic suites. Academic research labs and corporate R&D centers demand customizable instruments that support specialized protocols, while sports rehab centers seek platforms tailored to performance analytics.
Finally, distribution dynamics encompass both offline pathways-direct sales arrangements and authorized distributors-and online channels like e-commerce platforms and manufacturer websites. This dual-channel ecosystem enables market participants to reach diverse buyer segments, balancing the personalized service of direct engagement with the convenience and scalability of digital storefronts.
This comprehensive research report categorizes the Medical Dynamometer market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Technology
- Product Type
- Connectivity
- Application
- End User
- Distribution Channel
Exploring Key Regional Dynamics and Market Drivers Shaping Medical Dynamometer Adoption Across the Americas, Europe Middle East Africa and Asia Pacific
The Americas continue to exhibit strong dynamometer uptake driven by robust healthcare infrastructure and widespread research initiatives. In North America, high per-capita healthcare expenditure and an aging population foster consistent demand for musculoskeletal assessment tools in both clinical and home-care settings. Latin American markets, while characterized by budget constraints, are emerging as growth hotspots as telemedicine adoption accelerates and local manufacturers introduce cost-optimized solutions.
Across Europe, the Middle East and Africa, regulatory harmonization and cross-border collaborations are shaping device penetration. The European Union's Medical Device Regulation has heightened quality and safety standards, encouraging providers to procure certified dynamometers that align with stringent compliance criteria. Simultaneously, Gulf region investments in sports medicine and rehabilitation facilities are fueling demand for advanced force measurement systems, while African health initiatives are gradually integrating portable units to support community-based care programs.
In Asia-Pacific, rapid urbanization and escalating healthcare investments underpin a diverse market landscape. Developed economies such as Japan and Australia prioritize precision instruments for clinical research and specialized therapy, whereas emerging markets like India and Southeast Asia are witnessing increased adoption of affordable digital handheld models. Government-led initiatives to bolster domestic manufacturing are further amplifying local capacity, setting the stage for intensified competition and technology transfer throughout the region.
This comprehensive research report examines key regions that drive the evolution of the Medical Dynamometer market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Profiling Prominent Companies Driving Innovation, Strategic Collaborations and Competitive Dynamics within the Medical Dynamometer Industry Landscape
Leading manufacturers are spearheading innovation through targeted research and development efforts that refine sensor accuracy, device ergonomics and data analytics software. Some companies have introduced integrated platforms combining dynamometry with motion analysis, catering to comprehensive rehabilitation ecosystems. Others are forging strategic partnerships with academic institutions to validate new protocols and expand clinical evidence bases, reinforcing their market credibility.
Competitive landscapes are also being shaped by tiered product portfolios that address entry-level through high-end segments. Established players maintain dominance in specialized hospital and research channels with premium table-top instruments, while agile newcomers focus on digital handheld units optimized for telehealth and home-care settings. This dual-track approach facilitates both market retention and penetration into emerging end-user niches.
Beyond pure-play device manufacturers, collaborative ventures between medical equipment companies and software firms are accelerating the integration of cloud-based analytics and artificial intelligence. These alliances enable predictive modeling of patient progression, automated report generation and enhanced user interfaces, delivering value-added propositions that differentiate offerings and reinforce customer loyalty.
This comprehensive research report delivers an in-depth overview of the principal market players in the Medical Dynamometer market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Ametek, Inc.
- Fabrication Enterprises, Inc.
- Hausmann Enterprises, LLC
- Marsden Group
- MICROTEKNIK
- North Coast Medical Inc.
- Patterson Companies, Inc.
- Saehan Corporation
- Shimpo Instruments Co., Ltd.
- Takei Scientific Instruments Co., Ltd.
Actionable Recommendations Empowering Industry Leaders to Capitalize on Growth Opportunities and Navigate Challenges in the Evolving Medical Dynamometer Market
Invest in modular, software-centric platforms that support seamless firmware updates and third-party application integration to meet evolving clinical requirements. By prioritizing open-architecture design principles, device makers can foster developer ecosystems, accelerate feature enhancements and cultivate long-term customer engagement.
Diversify supply-chain portfolios by establishing relationships with multiple regional component suppliers and exploring near-shoring opportunities. This strategic flexibility mitigates exposure to tariff volatility and logistical disruptions, ensuring consistent device availability and cost stability.
Leverage advanced connectivity standards and cybersecurity protocols to reinforce interoperability with electronic health records and telemedicine services. Manufacturers should adopt encrypted communication channels, robust authentication mechanisms and remote maintenance capabilities to uphold data integrity and user trust.
Drive market expansion through targeted solutions for underserved segments such as home-based geriatric care and community-level rehabilitation. Customizing device form factors, user interfaces and service models for these niches can generate new revenue streams and enhance brand differentiation.
Cultivate strategic alliances with academic research centers and professional associations to validate device performance and establish clinical best practices. Co-developing evidence-based protocols and publishing peer-reviewed findings will amplify market credibility and support premium positioning.
Research Methodology Outlining Data Collection, Validation Processes and Analytical Frameworks Guiding the Medical Dynamometer Market Investigation
The research methodology underpinning this analysis integrates comprehensive secondary research and rigorous primary validation. Industry regulations, white papers and technical standards served as foundational references, while trade journals and patent filings supplemented insights into innovation trajectories. Market intelligence platforms provided contextual data, ensuring the research drew upon diverse, reputable sources.
Primary research was conducted through structured interviews and surveys with stakeholders across the value chain, including clinicians, procurement managers, R&D engineers and distribution partners. These engagements facilitated a deep understanding of end-user needs, purchasing criteria and adoption barriers. Data triangulation methods were applied to reconcile divergent perspectives, enhancing the reliability and robustness of the findings.
Quantitative inputs were validated through cross-referencing official trade statistics, customs databases and corporate financial disclosures. Qualitative assessments were corroborated by expert panels and advisory boards to ensure analytical rigor. An iterative review process refined key assumptions and harmonized terminology, resulting in a cohesive framework that accurately reflects current market dynamics and future inflection points.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Medical Dynamometer market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Medical Dynamometer Market, by Technology
- Medical Dynamometer Market, by Product Type
- Medical Dynamometer Market, by Connectivity
- Medical Dynamometer Market, by Application
- Medical Dynamometer Market, by End User
- Medical Dynamometer Market, by Distribution Channel
- Medical Dynamometer Market, by Region
- Medical Dynamometer Market, by Group
- Medical Dynamometer Market, by Country
- United States Medical Dynamometer Market
- China Medical Dynamometer Market
- Competitive Landscape
- List of Figures [Total: 18]
- List of Tables [Total: 2862 ]
Conclusion Emphasizing Critical Takeaways, Strategic Implications and Future Outlook for Stakeholders in the Medical Dynamometer Sector
This executive summary has highlighted the pivotal role of technological innovation, connectivity and strategic supply-chain management in shaping the medical dynamometer landscape. By dissecting segmentation nuances and regional dynamics, it has underscored the multifaceted drivers that influence device adoption across clinical, research and sports medicine settings.
The analysis of 2025 tariff impacts illustrated the imperative for agile sourcing strategies, material engineering innovations and collaborative manufacturing models. Simultaneously, segmentation insights provided clarity on the performance attributes and form factors most valued by distinct user groups, guiding targeted product development and marketing approaches.
Looking ahead, the fusion of AI-driven analytics, cloud-based platforms and remote monitoring capabilities is expected to redefine the value proposition of dynamometric assessment. Stakeholders who proactively embrace modular design, data interoperability and evidence-based validation will be best positioned to capture emerging opportunities. In this environment, continuous collaboration, regulatory alignment and investment in advanced R&D will serve as cornerstones of sustainable success.
Take Action Today to Gain Exclusive Insights and Empower Your Strategic Decisions with the Comprehensive Medical Dynamometer Market Report
Unlock unparalleled competitive advantage by acquiring this comprehensive market research report. Ketan Rohom, Associate Director of Sales & Marketing, stands ready to guide you through an in-depth exploration of market dynamics, segmentation insights, regional trends and strategic recommendations. Engaging with this report empowers your organization to make confident, data-driven decisions and seize emerging opportunities in the medical dynamometer arena.
Reach out today to secure your copy and embark on a transformative journey toward stronger market positioning, optimized product portfolios and sustainable growth. Harness actionable intelligence, leverage expert analysis and partner with Ketan Rohom to elevate your strategic roadmap within this rapidly evolving landscape.

- How big is the Medical Dynamometer Market?
- What is the Medical Dynamometer Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




