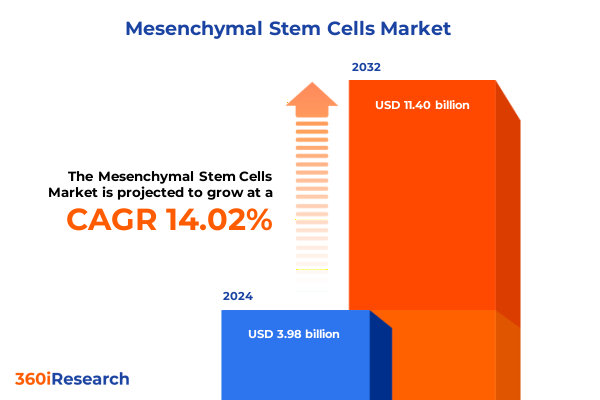
The Mesenchymal Stem Cells Market size was estimated at USD 3.96 billion in 2025 and expected to reach USD 4.81 billion in 2026, at a CAGR of 22.49% to reach USD 16.41 billion by 2032.
Unveiling the transformative potential and critical milestones shaping the mesenchymal stem cells arena to empower stakeholders with foundational insights
The expanding horizon of regenerative medicine has spotlighted mesenchymal stem cells (MSCs) as a pivotal therapeutic modality with the power to revolutionize patient care across a spectrum of indications. Over the past decade, breakthroughs in isolation techniques, expansion protocols, and targeted delivery systems have elevated MSCs from experimental curiosities to clinical candidates with the promise of addressing previously intractable disorders. As scientific understanding deepens, the field has shifted from rudimentary proof-of-concept studies to sophisticated trials that interrogate cellular behavior in complex tissue environments. Consequently, stakeholders across pharmaceutical development, academic research, and healthcare delivery are seeking coherent insights to navigate this dynamic ecosystem. This introduction sets the stage for a nuanced exploration of the forces reshaping the MSC landscape and illuminates key imperatives for informed decision-making.
Revolutionary advances in culture technologies and data-driven platforms are reshaping the mesenchymal stem cells research and development landscape
In recent years, the mesenchymal stem cells domain has witnessed transformative shifts driven by technological innovation and converging research disciplines. Advances in three-dimensional culture systems and bioreactor miniaturization have enabled more predictive in vitro modeling of therapeutic efficacy, while machine learning algorithms now guide cell selection and quality control with unprecedented precision. Breakthroughs in surface marker identification and single-cell analysis have refined our understanding of MSC heterogeneity, fostering the development of next-generation engineered products with enhanced potency and predictability. Moreover, groundbreaking partnerships between biotech firms and academic consortia are accelerating translational pipelines, bridging the gap between preclinical findings and clinical validation. These paradigm shifts underscore a maturation of the field, where integrated platforms and data-driven methodologies are redefining the metrics of success for MSC-based therapies.
Navigating the multifaceted effects of new US tariffs on bioprocessing components driving localized resilience and strategic supply chain recalibration
The imposition of new US tariffs on critical reagents and equipment in early 2025 has exerted a multifaceted impact on the mesenchymal stem cells sector. Heightened duties on imported bioreactor components and specialty cytokines have inflated production costs for research and manufacturing, compelling organizations to reassess their supply chain strategies. In response, a growing number of companies are forging domestic partnerships to insource critical inputs, thereby mitigating tariff-induced budgetary pressures. Simultaneously, the regulatory environment has adjusted to balance cost containment with patient safety, streamlining approvals for locally manufactured reagents that meet stringent quality standards. As a result, the ecosystem is evolving toward greater regional resilience, with stakeholders recalibrating procurement frameworks and investing in localized manufacturing capacities to fortify operations against future trade policy volatility.
Deconstructing advanced segmentation layers to illuminate distinct therapeutic, source, and product type trajectories in the mesenchymal stem cells landscape
A granular examination of mesenchymal stem cells market segmentation reveals nuanced growth drivers across therapeutic applications and research modalities. Applications within cardiology, neurology, and orthopedics continue to attract substantial investment, driven by robust preclinical evidence of MSC-mediated tissue repair and immunomodulation in autoimmune disorders and wound healing. Simultaneously, differentiation potential sourced from adipose tissue and bone marrow remains a benchmark for product developers, although emerging interest in umbilical cord and dental pulp sources reflects a shift toward more ethically accessible and potent cell populations. End users such as hospitals and clinics are optimizing their procedural workflows by leveraging turnkey services offered by contract research organizations and specialized cell banks. Moreover, the demand for instruments and accessories complements growth in kits and reagents, where enzymatic dissociation reagents and media supplements are critical for standardized cell expansion. Culture methods are diversifying: stirred tank bioreactors support scale-up efforts, while hydrogel-based three-dimensional cultures are gaining traction for their ability to mimic physiological niches. This complexity is further accentuated by the distinction between allogeneic and autologous therapies, which dictates development timelines and commercialization strategies.
This comprehensive research report categorizes the Mesenchymal Stem Cells market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Application
- Source
- End User
- Product Type
- Culture Method
- Therapy Type
Examining how divergent regulatory frameworks and funding landscapes across Americas, Europe Middle East Africa, and Asia Pacific are sculpting market growth
Regional dynamics continue to shape the trajectory of the MSC market, driven by divergent regulatory frameworks, funding priorities, and healthcare infrastructure. In the Americas, strong government support for cell therapy research and a mature clinical trial ecosystem have fostered rapid adoption of innovative protocols, with leading research institutes and biotech hubs driving early-stage proof-of-concept studies. Meanwhile, Europe, the Middle East, and Africa present a heterogeneous landscape where regulatory harmonization efforts under the European Medicines Agency coexist with localized initiatives in regenerative medicine, particularly in the Gulf region and select African nations. Asia-Pacific, by contrast, is defined by aggressive public and private investments, with several countries enacting streamlined approval pathways and offering incentives to attract foreign direct investment in cell manufacturing facilities. These regional contrasts underscore the importance of geographic diversification for market participants seeking to balance risk and opportunity across regulatory and economic environments.
This comprehensive research report examines key regions that drive the evolution of the Mesenchymal Stem Cells market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Highlighting strategic collaborations and proprietary innovations that differentiate market leaders in the mesenchymal stem cells sector
Leading players in the mesenchymal stem cells arena are distinguished by their integrated value chains and robust patent portfolios. Firms pioneering proprietary expansion systems and automated bioreactor platforms have established strategic partnerships with clinical networks to expedite translational studies. Concurrently, emerging innovators are challenging incumbents by focusing on niche applications, such as immunomodulation in inflammatory bowel disease or neuroregeneration following traumatic brain injury. Collaborations between contract research organizations and cellular therapy developers are yielding hybrid service models that combine process optimization with clinical trial management. Furthermore, alliances between reagent suppliers and instrument manufacturers are streamlining workflow integration, enabling end users to achieve reproducible outcomes. Collectively, these strategic maneuvers are intensifying competitive dynamics, driving continuous improvement in product quality and operational efficiency.
This comprehensive research report delivers an in-depth overview of the principal market players in the Mesenchymal Stem Cells market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Anterogen Co., Ltd.
- Athersys, Inc.
- Cellular Biomedicine Group, Inc.
- Cynata Therapeutics Limited
- Lonza Group AG
- Mesoblast Limited
- Pluristem Therapeutics Inc.
- SanBio Co., Ltd.
- STEMCELL Technologies Inc.
- Vericel Corporation
Implementing a comprehensive innovation and operational excellence framework to secure competitive advantage in the mesenchymal stem cells market
To capitalize on the burgeoning opportunities in mesenchymal stem cells, industry leaders should pursue a multi-pronged approach that integrates innovation with operational excellence. Stakeholders must prioritize investment in scalable bioprocessing platforms and modular manufacturing facilities to accommodate both autologous and allogeneic workflows. Rigorous engagement with regulatory authorities early in development can streamline product approvals and foster mutual understanding of novel assay requirements. Strategic partnerships with academic institutions and contract research organizations can accelerate translational pipelines while ensuring access to specialized expertise and patient cohorts. Additionally, companies should establish robust supply chain risk management frameworks, emphasizing diversified sourcing of critical reagents and components. By aligning these initiatives with clear corporate objectives and transparent communication, organizations can navigate market complexities and position themselves for sustainable growth in the evolving MSC ecosystem.
Detailing a rigorous multi-dimensional research methodology combining primary insights and exhaustive secondary analysis for robust market intelligence
This market research report integrates primary and secondary research methodologies to ensure comprehensive coverage of the mesenchymal stem cells landscape. Primary research comprised in-depth interviews with senior executives across pharmaceutical companies, biotech firms, contract research organizations, and academic institutions to capture firsthand insights into strategic priorities and operational challenges. Secondary research involved exhaustive reviews of peer-reviewed journals, regulatory filings, patents, and industry conferences to validate market trends and technological advancements. Quantitative analysis was employed to assess volume-based metrics within bioprocessing workflows, while qualitative assessments illuminated product differentiation and competitive positioning. Data triangulation was applied at every stage to enhance accuracy, supported by a cross-functional team of domain experts in cell biology, bioengineering, and market analytics. The result is a robust, multi-dimensional view that empowers stakeholders with actionable intelligence.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Mesenchymal Stem Cells market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Mesenchymal Stem Cells Market, by Application
- Mesenchymal Stem Cells Market, by Source
- Mesenchymal Stem Cells Market, by End User
- Mesenchymal Stem Cells Market, by Product Type
- Mesenchymal Stem Cells Market, by Culture Method
- Mesenchymal Stem Cells Market, by Therapy Type
- Mesenchymal Stem Cells Market, by Region
- Mesenchymal Stem Cells Market, by Group
- Mesenchymal Stem Cells Market, by Country
- United States Mesenchymal Stem Cells Market
- China Mesenchymal Stem Cells Market
- Competitive Landscape
- List of Figures [Total: 18]
- List of Tables [Total: 1590 ]
Synthesis of key insights revealing how technological innovation, policy shifts, and strategic partnerships are converging to advance mesenchymal stem cell therapies
In conclusion, mesenchymal stem cells stand at the forefront of regenerative medicine, offering versatile therapeutic platforms that address critical unmet medical needs. Technological advancements in culture systems, data analytics, and cell sourcing are driving a maturation of the field, while evolving regulatory landscapes and policy interventions are shaping regional growth trajectories. The cumulative impact of recent trade policies underscores the necessity for adaptable supply chains and localized manufacturing strategies. Granular segmentation across applications, sources, and product modalities reveals diverse pathways for innovation, and strategic partnerships between industry incumbents and emerging players are intensifying competitive dynamics. By adopting a holistic approach that blends scientific rigor with operational agility, stakeholders can harness the full potential of MSC-based therapies to deliver transformative health outcomes.
Secure your comprehensive mesenchymal stem cells market research report today by engaging with Associate Director Sales & Marketing Ketan Rohom
To unlock a comprehensive understanding of the mesenchymal stem cells market and its strategic implications, reach out directly to Associate Director, Sales & Marketing, Ketan Rohom to secure your detailed market research report today. This report delivers actionable intelligence, tailored recommendations, and rigorous analysis to support critical decision-making. Engage with Ketan Rohom to elevate your market positioning, leverage emerging opportunities, and align your organization’s initiatives with the latest innovations in regenerative medicine. Don’t let this opportunity slip away-connect with Ketan Rohom now to empower your team with the insights needed to thrive in a rapidly evolving landscape.

- How big is the Mesenchymal Stem Cells Market?
- What is the Mesenchymal Stem Cells Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




