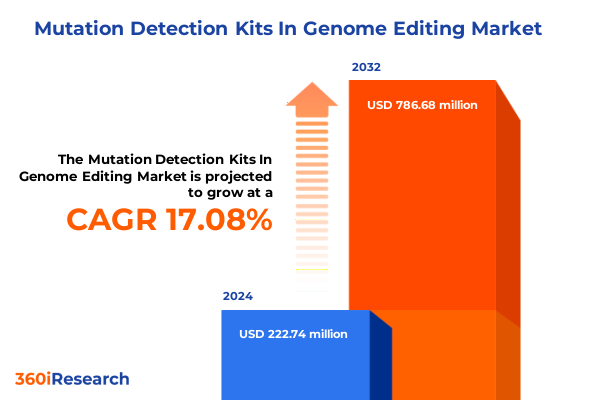
The Mutation Detection Kits In Genome Editing Market size was estimated at USD 259.19 million in 2025 and expected to reach USD 303.32 million in 2026, at a CAGR of 17.18% to reach USD 786.68 million by 2032.
Pioneering Precision in Genome Editing Through Advanced Mutation Detection Kits Illuminates the Pathway to Unmatched Biotech Innovation and Safety
Genome editing has ushered in a new era of precision biotechnology, with mutation detection kits playing a pivotal role in verifying the accuracy and safety of targeted genetic modifications. These kits serve as essential tools for characterizing on-target and off-target edits introduced by nucleases, enabling researchers to confirm that desired alterations have been made without unintended genetic changes. As researchers refine their editing protocols, from early-stage discovery to clinical applications, the ability to rapidly and reliably detect mutations becomes indispensable for ensuring regulatory compliance and advancing therapeutic breakthroughs.
In recent years, the evolution of mutation detection methodologies has been marked by a shift from traditional single-assay approaches toward integrated platforms that combine high-throughput sequencing with advanced bioinformatic analysis. This convergence delivers greater sensitivity and specificity, empowering scientists to discern even low-frequency variants within complex genomic backgrounds. Consequently, laboratories can accelerate development timelines by reducing the need for iterative validation steps.
Moreover, as genome editing extends beyond basic research into fields such as precision medicine, agricultural biotechnology, and cell therapy, mutation detection kits have adapted to meet diverse end-user requirements. From benchtop assays optimized for rapid turnaround to fully automated, high-throughput workflows, these platforms cater to a wide range of throughput, cost, and performance demands. Collectively, these innovations are setting new benchmarks for accuracy and reproducibility in the genome editing landscape, underpinning the next wave of genetic research and application.
Revolutionary Developments in Mutation Detection Technologies Catalyzing Unprecedented Advances Across Genome Editing Paradigms
The landscape of mutation detection in genome editing is undergoing transformative shifts driven by breakthroughs in nuclease engineering, sequencing technologies, and computational analysis. The advent of next-generation base editors and prime editors has introduced tools capable of single-base modifications without double-stranded breaks, necessitating detection platforms that can accurately identify subtle nucleotide changes. In response, assay providers have developed tailored chemistries and workflows that specifically capture these unique editing signatures, empowering researchers to harness base editing’s therapeutic potential with confidence.
Concurrently, the integration of high-fidelity nuclease variants has significantly minimized off-target effects, prompting detection kits to emphasize ultra-sensitive identification of rare events. The deployment of error-corrected sequencing methods and molecular barcoding techniques further elevates the ability to distinguish true edits from PCR-induced artifacts. As a result, laboratories can now achieve depth and accuracy levels that were previously unattainable, facilitating the translation of genome editing applications into clinical pipelines.
These technical leaps are complemented by advances in artificial intelligence and machine learning algorithms, which streamline the analysis of complex sequencing datasets. Automated variant-calling pipelines not only accelerate data interpretation but also standardize reporting formats, reinforcing reproducibility across research institutions. Taken together, these converging developments are redefining the standards for mutation detection, cementing its role as a cornerstone technology that accelerates innovation across therapeutic, agricultural, and industrial genome editing applications.
Evaluating the Cumulative Impacts of 2025 U.S. Tariff Policies on the Global Movement of Genome Editing Mutation Detection Kits
The cumulative effect of U.S. tariff policies enacted in 2025 has introduced significant cost pressures and supply chain complexities within the mutation detection kit market. Beginning in April, the implementation of a global 10% import tariff on most goods entering the United States encompassed critical reagents, consumables, and instrumentation essential for genome editing assays, driving procurement costs upward for research institutions and biopharma companies alike. Subsequently, targeted tariffs under Section 301 on imports from China and the European Union imposed additional duties, reaching rates of 30% and 20%, respectively, on specialized life science tools and sequencing platforms.
These layered duties have compelled kit manufacturers to reevaluate sourcing strategies, prompting initiatives to diversify manufacturing footprints or qualify alternative suppliers. Several Chinese-based reagent producers have accelerated domestic stockpiling and explored local production collaborations to mitigate tariff-driven disruptions and shield clients from price volatility. Meanwhile, U.S. government extensions of exclusions for select biotech inputs under the Section 301 framework provided temporary relief through August 2025, though expiration timelines continue to generate strategic uncertainty for R&D planning.
The combined impact of these tariff measures is manifested in elevated cost of goods sold and extended lead times for key assay components. Early-stage biotech startups and academic laboratories, which operate under constrained budgets and tight timelines, face heightened risk of delayed project milestones. Conversely, large-scale pharmaceutical and CRO partners are investing in onshoring critical reagent production and negotiating tariff-pass-through clauses to sustain profitability margins. In aggregate, the 2025 tariff landscape underscores the imperative for dynamic supply chain resilience in the rapidly evolving mutation detection kit market.
Deep Dive into Multidimensional Segmentation Reveals Strategic Insights for Targeting Mutation Detection Kit Markets Across Technologies and Applications
Insight into market segmentation reveals nuanced opportunities and challenges across multiple dimensions of the mutation detection kit ecosystem. When examining the technology dimension, Crispr systems dominate with subdivided focus on Cas9 for classical gene disruption, Cas12 for editing precision enhancements, and Cas13 for RNA-targeted applications. Meanwhile, specialist platforms based on meganucleases, TALENs, and ZFNs continue to serve niche applications requiring bespoke editing profiles with unique target specificities.
Considering the methods of detection, high-resolution melt analysis remains favored for its rapid, cost-effective screening capabilities, whereas next-generation sequencing has expanded through amplicon, targeted, and whole-genome approaches to capture comprehensive variant landscapes. PCR-based assays have likewise diversified, encompassing digital PCR for absolute quantification, end point PCR for straightforward detection, and real-time PCR for dynamic reaction monitoring.
Applications drive distinct purchasing patterns, with agricultural biotechnology initiatives prioritizing throughput and robustness, basic research labs seeking flexibility and method validation, clinical diagnostics demanding regulatory compliance and traceability, drug discovery teams emphasizing sensitivity and multiplexing, and personalized medicine projects requiring bespoke, patient-centric workflows. End users range from academic and research institutes to clinical diagnostic laboratories, with contract research organizations bridging service-intensive projects and pharmaceutical and biotechnology companies anchoring long-term development pipelines.
Distribution channels influence market reach and support structures, as direct sales offer enhanced customer engagement, distributors provide regional coverage, online channels ensure rapid access to consumables, and original equipment manufacturers embed detection modules within integrated instrument suites. Finally, workflow stages delineate value add, from pre-editing quality control through post-editing validation to downstream data analysis, highlighting the importance of end-to-end solutions tailored to specific procedural steps.
This comprehensive research report categorizes the Mutation Detection Kits In Genome Editing market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Technology
- Detection Method
- Workflow Stage
- Application
- End User
- Distribution Channel
Regional Dynamics Uncovered Across Americas, EMEA, and Asia-Pacific Highlight Varied Growth Motivators and Regulatory Landscapes in Genome Editing Kits
Regional dynamics exert a profound influence on the adoption and evolution of mutation detection kits, shaped by regulatory frameworks, infrastructural maturity, and localized research priorities. In the Americas, robust funding for gene therapies and agricultural biotech has catalyzed the deployment of cutting-edge detection platforms, while progressive regulatory pathways accelerate clinical validation protocols. Established reagent suppliers and instrument manufacturers maintain strong distribution networks, yet face emerging competition from innovative startups in Canada and Latin America that emphasize cost-effective, portable assay formats.
Across Europe, the Middle East, and Africa, diverse regulatory environments and varying levels of research infrastructure drive differential kit preferences. Western European markets, buoyed by significant public-private funding partnerships, gravitate toward automated, high-throughput solutions suited for large-scale clinical and diagnostic settings. In contrast, Middle Eastern initiatives leverage public investments in genomic medicine, fostering collaborations with local academic centers. Meanwhile, sub-Saharan Africa’s growing emphasis on infectious disease surveillance and agricultural resilience is beginning to adopt smaller-scale, field-deployable mutation detection assays to address region-specific challenges.
The Asia-Pacific region exhibits one of the fastest adoption rates for genome editing and associated detection technologies, underpinned by extensive government-sponsored genomics initiatives in countries such as China, Japan, South Korea, and India. Local manufacturing capabilities and favorable trade policies bolster the domestic supply of reagents and kits, while multinational partnerships facilitate technology transfer. Collectively, these factors create a dynamic landscape where regional innovation hubs quickly translate novel detection chemistries into commercial offerings, setting the stage for sustained growth and cross-border collaborations.
This comprehensive research report examines key regions that drive the evolution of the Mutation Detection Kits In Genome Editing market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Comprehensive Profiling of Leading Mutation Detection Kit Manufacturers Reveals Strategic Positioning, R&D Leadership, and Market Differentiation
An analysis of the competitive landscape identifies leading and emerging players shaping the mutation detection kit market through strategic investments, product innovation, and collaborative partnerships. Established life science suppliers have bolstered their portfolios by integrating advanced detection chemistries and robust software solutions into cohesive offerings. Concurrently, specialized reagent companies are differentiating through proprietary assay designs that deliver ultra-sensitive variant identification, catering to high-value clinical and therapeutic applications.
Collaborative ventures between instrumentation providers and informatics firms have yielded turnkey platforms that streamline end-to-end workflows, reducing the technical burden on laboratory personnel. Several start-up enterprises have entered the arena with disruptive technologies, such as microfluidic-based detection modules and single-molecule sequencing integration, challenging incumbents to enhance performance and user experience. Ecosystem partnerships with contract research organizations and academic consortia further extend market reach and accelerate validation studies across diverse genomic contexts.
Investment trends signal intensifying focus on artificial intelligence-driven data interpretation tools that complement wet-lab detection assays, enabling real-time variant calling and predictive modeling of editing outcomes. Strategic alliances and licensing agreements underscore the importance of integrated solutions that address both hardware and software requirements. As consolidation activities continue, companies possessing comprehensive product suites-from pre-editing quality control assays through post-editing data analysis-are poised to capture substantial value from end-to-end service offerings in the mutation detection market.
This comprehensive research report delivers an in-depth overview of the principal market players in the Mutation Detection Kits In Genome Editing market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Agilent Technologies, Inc.
- Amoy Diagnostics Co., Ltd.
- Applied Biological Materials, Inc.
- Bio-Rad Laboratories
- BIOKÉ, B.V. by Cell Signaling Technology, Inc.
- Bioneer Corporation
- Biovision Inc. by Abcam Limited
- GenScript Biotech Corporation
- Illumina, Inc.
- Integrated DNA Technologies, Inc. by Danaher Corporation
- Launch Diagnostics Limited by Avacta Group Plc
- LGC Biosearch Technologies
- Lonza Group AG
- Medaysis Company
- Merck KGaA
- Mylab Discovery Solutions Pvt. Ltd.
- New England Biolabs
- New England Biolabs, Inc.
- Origene Technologies, Inc.
- Promega Corporation
- Qiagen N.V.
- Synthego Corporation
- Takara Bio Inc.
- Thermo Fisher Scientific Inc.
- TransGen Biotech Co., Ltd.
- TRUPCR by Kilpest India Limited
Strategic Imperatives for Industry Leaders to Navigate Evolving Technical, Regulatory, and Supply Chain Challenges in Mutation Detection Kits
To maintain a sustainable competitive advantage, industry leaders should prioritize the development of modular detection platforms that seamlessly integrate with diverse genome editing workflows. Embracing flexible licensing models for software updates and assay expansions can further drive adoption by reducing entry barriers for emerging research groups. Strategic investments in onshore manufacturing or regional supply partnerships will mitigate tariff exposure and enhance supply chain resilience, especially in light of evolving trade policies.
Additionally, fostering collaborative networks with academic and clinical research institutions can accelerate the validation of novel detection methodologies. Co-development programs that focus on unmet needs in agricultural and industrial biotechnology applications will broaden market applicability. Combining assay development with real-time data analytics capabilities can streamline user workflows, reducing turnaround times and operational complexity.
Leaders should also engage proactively with regulatory agencies to harmonize validation standards and expedite approval pathways. Cultivating interoperability between detection kits and existing laboratory information management systems will enhance user convenience and drive preference for integrated solutions. Finally, channeling resources into customer training, technical support, and flexible distribution strategies will strengthen end-user relationships and unlock new market segments across emerging geographies.
Robust Research Framework Integrating Primary and Secondary Insights Underpins Comprehensive Analysis of Mutation Detection Kits in Genome Editing
Our research synthesis combines primary interviews, secondary literature review, and quantitative data analysis to deliver robust insights into mutation detection kit market dynamics. Primary research included in-depth discussions with key opinion leaders across academic, clinical, and industrial laboratories, ensuring a holistic perspective on unmet needs and adoption drivers. Secondary research encompassed examination of peer-reviewed journals, regulatory filings, and publicly available company reports to validate technological advancements and competitive positioning.
Quantitative segmentation analysis leveraged a proprietary database tracking product launches, partnership announcements, and regional distribution trends. Methodological rigor was applied through cross-validation of data sources and triangulation of findings to minimize bias. Workflow-stage mapping integrated feedback from bench scientists to prioritize critical validation steps, while tariff impact assessments utilized trade policy documents and industry surveys to contextualize cost and supply chain considerations through 2025.
This iterative approach ensures that our findings reflect current market realities and anticipate near-term shifts. Rigorous quality control protocols, including statistical coherence checks and expert review panels, underpin the reliability of our conclusions. The combination of qualitative insights and quantitative metrics provides a comprehensive understanding of the mutation detection kit ecosystem, enabling stakeholders to make informed strategic decisions.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Mutation Detection Kits In Genome Editing market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Mutation Detection Kits In Genome Editing Market, by Technology
- Mutation Detection Kits In Genome Editing Market, by Detection Method
- Mutation Detection Kits In Genome Editing Market, by Workflow Stage
- Mutation Detection Kits In Genome Editing Market, by Application
- Mutation Detection Kits In Genome Editing Market, by End User
- Mutation Detection Kits In Genome Editing Market, by Distribution Channel
- Mutation Detection Kits In Genome Editing Market, by Region
- Mutation Detection Kits In Genome Editing Market, by Group
- Mutation Detection Kits In Genome Editing Market, by Country
- United States Mutation Detection Kits In Genome Editing Market
- China Mutation Detection Kits In Genome Editing Market
- Competitive Landscape
- List of Figures [Total: 18]
- List of Tables [Total: 1590 ]
Concluding Perspectives on the Future Trajectory of Mutation Detection Kits Shaping Genome Editing Efficacy and Biotech Innovation
Mutation detection kits have emerged as foundational components in the genome editing toolkit, enabling precise characterization of editing events and fostering confidence in both research and clinical settings. Technological innovations, from enhanced nuclease specificity to advanced sequencing workflows, have elevated the accuracy and throughput of detection assays, driving broader adoption across diverse applications.
Market segmentation analysis highlights the importance of tailored solutions across technology types, detection methods, applications, and end-user scenarios. Regional insights reveal that while mature markets prioritize automation and regulatory compliance, emerging regions focus on cost-effective and field-ready platforms. Moreover, tariff developments in 2025 underscore the necessity for adaptive supply chain strategies that balance global sourcing with onshore resilience.
Looking ahead, the convergence of wet-lab assay innovation with artificial intelligence-driven analytics will define the next wave of performance improvements. Industry partnerships and regulatory collaborations will further streamline validation pathways, reinforcing the role of mutation detection kits in accelerating genome editing’s translation from bench to bedside. As the field continues to evolve, stakeholders equipped with comprehensive market insights will be best positioned to harness emerging opportunities and navigate potential challenges.
Unlock In-Depth Insights and Elevate Strategic Planning by Engaging with Ketan Rohom for Your Comprehensive Mutation Detection Kit Market Report
For stakeholders eager to deepen their strategic understanding and secure a competitive edge, Ketan Rohom is the ideal partner to guide your market intelligence journey. As Associate Director of Sales & Marketing, Ketan brings a wealth of expertise in translating complex genomic data into actionable business strategies. Engaging directly with Ketan ensures personalized insights tailored to your organization’s unique needs, whether you are refining product development roadmaps or optimizing market entry strategies.
By initiating a consultation with Ketan Rohom, you gain access to in-depth analysis, proprietary data segments, and expert recommendations that illuminate opportunity areas across technologies, applications, and geographies. This direct collaboration accelerates your decision-making process and aligns your strategic planning with the most current industry dynamics. Invest in a partnership that transforms comprehensive market research into a catalyst for your growth. Reach out to Ketan Rohom today to secure your copy of the full Mutation Detection Kits in Genome Editing market research report and embark on the next phase of your innovation journey.

- How big is the Mutation Detection Kits In Genome Editing Market?
- What is the Mutation Detection Kits In Genome Editing Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




