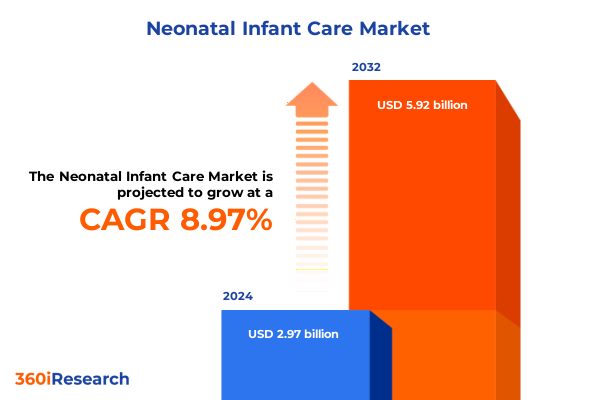
The Neonatal Infant Care Market size was estimated at USD 3.24 billion in 2025 and expected to reach USD 3.54 billion in 2026, at a CAGR of 8.96% to reach USD 5.92 billion by 2032.
Pioneering the Future of Neonatal Infant Care through Interdisciplinary Collaboration, Compassionate Practices, and Cutting-Edge Technologies
The neonatal infant care landscape is evolving at an unprecedented pace, driven by converging forces across technology, clinical practice, and policy domains. Recent advancements in digital health platforms have fostered real-time data exchange and remote monitoring capabilities, enabling clinicians to deliver personalized interventions with greater efficiency and precision. At the same time, heightened global attention to neonatal outcomes has spurred collaborative initiatives among healthcare providers, academic researchers, and device manufacturers to refine evidence-based protocols and accelerate the translation of innovations from bench to bedside.
This introduction provides essential context for understanding the dynamic market forces shaping neonatal infant care devices. It outlines the critical intersections of clinical imperatives-such as reducing the risks of preterm birth complications and improving thermoregulation stability-with emerging solutions like AI-driven diagnostic support, modular device architectures, and service-based provisioning models. By framing these developments within the broader healthcare ecosystem, decision-makers can appreciate how strategic investments in neonatal technologies align with overarching goals of operational resilience, regulatory compliance, and patient-centric quality improvement.
Through a synthesis of recent industry developments, regulatory updates, and clinical research, this section sets the stage for an in-depth exploration of transformative shifts, tariff impacts, segmentation strategies, regional dynamics, and actionable recommendations that form the backbone of this executive summary. It equips stakeholders with a clear understanding of the foundational trends and challenges that will guide strategic planning in neonatal infant care across 2025 and beyond.
Unveiling the Transformative Technological, Clinical, and Policy Shifts Reshaping Neonatal Infants’ Care Delivery and Outcomes
The neonatal care ecosystem is undergoing transformative shifts driven by the seamless integration of intelligent technologies, evolving clinical protocols, and the emergence of hybrid care models. Artificial intelligence embedded within respiratory care devices now analyzes respiratory waveforms and apnea trends in real time, delivering automated ventilator adjustments and predictive maintenance alerts to clinicians. Early adopters report significant reductions in false alarms and unscheduled downtime, illustrating how data-driven insights can enhance workflow efficiency and patient safety.
Simultaneously, tele-NICU frameworks have expanded the reach of specialized neonatal expertise to remote and underserved regions. Connected ventilators and wearable sensors now stream critical vital signs to centralized hub hospitals, enabling proactive clinical interventions without the need for patient transfers. This model has demonstrated earlier detection of respiratory deterioration and reduced transfer-related stress on neonates and families.
In parallel, device miniaturization and modular design principles are reshaping procurement strategies across NICUs. Compact multifunctional units that combine radiant warmers with integrated monitoring platforms optimize limited floor space and reduce handling risks. The rise of service-based leasing and subscription models for phototherapy and infusion equipment is unlocking access for smaller clinics and home care providers, aligning capital expenditure with utilization patterns and improving cost predictability.
Finally, sustainability and environmental stewardship have emerged as key priorities. Manufacturers are introducing reusable consumables, biodegradable components, and energy-efficient designs to help healthcare institutions meet carbon reduction targets while maintaining clinical performance. Together, these technological, operational, and policy shifts are redefining best practices in neonatal infant care and setting new benchmarks for quality, efficiency, and equity.
Assessing the Cumulative Effects of United States Tariff Adjustments on Critical Neonatal Infant Care Devices Throughout 2025
Throughout 2025, the cumulative effect of U.S. tariff adjustments under the Section 301 framework has significantly influenced the import and pricing dynamics of critical neonatal infant care devices. On January 1, 2025, tariffs on semiconductors used in monitoring modules increased from 25% to 50%, while surgical and non-surgical respirators-including certain ventilator components-rose from 0–7.5% to 25%, with a subsequent escalation to 50% scheduled for January 1, 2026. Concurrently, tariffs on disposable medical gloves and masks climbed to 50%, exerting pressure on hospitals’ supply chains and cost structures.
Of particular concern to NICU teams is the forthcoming 100% tariff on syringes and needles, excluding enteral feeding syringes until 2026, which threatens to disrupt procurement processes for infusion pumps and related consumables. The American Hospital Association has underscored the risk that shifting to non-enteral products could compromise patient safety by replacing specialized ISO-compliant feeding syringes with standard alternatives that lack critical safety features.
Far from a theoretical issue, these tariff hikes have already prompted major equipment providers to reconsider global manufacturing footprints, inventory buffers, and supplier diversification strategies. They have also heightened advocacy efforts for targeted exemptions on humanitarian-impact goods. As a result, procurement leaders face the twin challenges of mitigating short-term budgetary shocks and recalibrating long-term sourcing plans to safeguard continuity of care in neonatal settings.
Uncovering Key Segmentation Insights to Refine Neonatal Infant Care Device Strategies Across Product, End User, Channel, Application, and Therapeutic Dimensions
Analyzing the market through a product-type lens reveals that infusion pumps, subdivided into syringe and volumetric categories, must balance precision dosing needs against evolving tariff-driven consumable costs. Neonatal warmers-which comprise infant incubators and radiant warmers-are increasingly evaluated on energy efficiency and integration with digital monitoring ecosystems. Phototherapy devices, spanning fiber-optic, fluorescent, and LED technologies, are differentiated by treatment efficacy, ease of use, and opportunities for remote monitoring. Meanwhile, respiratory care devices, including CPAP systems, neonatal ventilators, and oxygen hoods, are assessed based on AI-enabled decision support, modular design, and interoperability with tele-NICU infrastructures.
From an end-user standpoint, hospitals-both private and public-continue to dominate capital investments in high-complexity NICU equipment, whereas ambulatory surgical centers, including freestanding surgery centers, and specialty clinics such as dedicated neonatal intensive care units leverage portable warmers and monitoring tools to extend care beyond traditional settings. Home care settings, supported by chartered providers and independent caregivers, are embracing subscription-based infusion and phototherapy solutions to facilitate earlier discharge and outpatient follow-up.
Distribution channels, including direct sales forces, domestic and international wholesalers, hospital supply companies, and online retail platforms-ranging from e-commerce marketplaces to manufacturer websites-are adapting to hybrid procurement models that combine strategic stocking agreements with just-in-time deliveries. In terms of application, intravenous therapy and temperature management solutions are undergoing convergence, with multifunction platforms offering infusion capabilities alongside active warming. Jaundice treatment spans conventional and LED phototherapy, while monitoring domains integrate blood gas analysis with vital signs surveillance. Respiratory therapy encompasses both invasive and non-invasive modalities, and therapeutic areas such as hypothermia, neonatal abstinence syndrome, jaundice management, respiratory distress syndrome, and sepsis interventions are driving specialized device requirements.
This comprehensive research report categorizes the Neonatal Infant Care market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Product Type
- Therapeutic Area
- Distribution Channel
- Application
- End User
Deriving Regional Insights from the Americas, EMEA, and Asia-Pacific to Inform Growth and Tailored Approaches in Neonatal Infant Care Markets
In the Americas, the neonatal infant care market is characterized by a well-established healthcare infrastructure, robust reimbursement frameworks, and high adoption rates of AI-enabled respiratory and monitoring technologies. Key initiatives led by public and private payers in the United States are incentivizing value-based care models that reward outcomes over volume, fostering pilot programs in tele-NICU networks and home transition services.
The Europe, Middle East & Africa region presents a complex mosaic of regulatory environments and funding mechanisms. Within Western Europe, stringent medical device regulations and sustainability mandates are accelerating the shift toward reusable consumables and eco-design principles. In contrast, emerging economies in the Middle East and parts of Africa are benefiting from donor-funded NICU expansion projects, which often prioritize cost-effective multifunctional incubators and solar-powered phototherapy units to address infrastructural challenges.
In the Asia-Pacific region, surging birth rates and expanding private healthcare investments are fueling demand for technologically advanced neonatal equipment. Manufacturers are responding by localizing production in India and Southeast Asia to mitigate lead times and tariff impacts, while tailored financing solutions and leasing structures support smaller hospitals and clinics. Across the region, public-private partnerships and government-backed neonatal care initiatives are instrumental in scaling up access to critical life-saving devices.
This comprehensive research report examines key regions that drive the evolution of the Neonatal Infant Care market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Highlighting Leading Industry Players Driving Innovation Across Neonatal Infant Care Devices with Strategic Partnerships and Technological Breakthroughs
Leading device manufacturers such as Medtronic have rolled out AI-augmented ventilator systems that deliver adaptive pressure support based on neonatal breathing patterns, reducing manual recalibrations and improving oxygenation stability. Dräger has introduced combined CPAP and humidification units optimized for use in mobile NICU settings, enhancing patient mobility and reducing equipment footprints. Hamilton Medical’s establishment of a local assembly plant in India reflects a broader strategic pivot to regionalized supply chains, which has shortened delivery cycles and improved after-sales service responsiveness.
Philips continues to expand its digital care management platforms with cloud-enabled phototherapy and monitoring suites that allow remote parameter adjustment and automated treatment logs. GE Healthcare is advancing its wearable neonatal monitoring sensors, enabling seamless integration with hospital EHR systems and parent-facing mobile applications. Fisher & Paykel Healthcare is at the forefront of modular warming systems that combine radiant warming with integrated vital signs tracking, catering to space-constrained NICU footprints.
Emerging players and joint ventures are also shaping the competitive landscape. Strategic collaborations between technology start-ups specializing in machine learning algorithms and established medtech firms are yielding next-generation closed-loop ventilation trials. Meanwhile, service-oriented entrants are offering outcome-based leasing agreements that align device performance metrics with reimbursement frameworks, challenging traditional capital procurement models.
This comprehensive research report delivers an in-depth overview of the principal market players in the Neonatal Infant Care market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Ambu A/S
- Atom Medical Corporation
- B. Braun Melsungen AG
- Becton, Dickinson and Company
- Cardinal Health, Inc.
- DeRoyal Industries
- Drägerwerk AG & Co. KGaA
- Fisher & Paykel Healthcare Corporation
- General Electric Company
- Getinge Group
- Hamilton Medical
- Inspiration Healthcare Group plc
- Koninklijke Philips N.V.
- Löwenstein Medical SE & Co. KG
- Masimo Corporation
- Medtronic plc
- Natus Medical Incorporated
- nice Neotech Medical Systems Pvt. Ltd.
- Nihon Kohden Corporation
- Phoenix Medical Systems
- ResMed, Inc.
- Smiths Group plc
- Teleflex Incorporated
- Utah Medical Products, Inc.
- Vyaire Medical
Delivering Actionable Recommendations for Industry Leaders to Capitalize on Emerging Trends and Strengthen Neonatal Infant Care Outcomes
Industry leaders aiming to thrive in this evolving environment should prioritize integrated technology roadmaps that encompass AI-driven decision support, interoperability, and data analytics. By forging strategic partnerships with clinical centers of excellence and digital health vendors, manufacturers can accelerate device validation studies and secure early market adoption.
To mitigate tariff-related cost pressures, organizations must diversify supplier networks, explore near-shoring options, and advocate for targeted exemptions on life-sustaining medical devices. In parallel, adopting service-based and outcome-linked commercial models can align cost structures with patient throughput and quality metrics, unlocking new revenue streams and fostering client loyalty.
Manufacturers and healthcare providers should also embed sustainability objectives into product design and procurement policies. Emphasizing reusable consumables, energy-efficient platforms, and circular economy principles will not only meet regulatory mandates but also resonate with environmental, social, and governance priorities among payers and hospital networks.
Finally, stakeholders should invest in workforce training and change management programs to ensure seamless integration of advanced technologies into clinical workflows. Cultivating digital literacy and data-driven mindsets within neonatal care teams will be essential to harness the full potential of next-generation devices and tele-NICU infrastructures.
Detailing the Comprehensive Research Methodology Employed for Rigorous Analysis and Validated Insights in Neonatal Infant Care Studies
This research synthesis draws upon a robust mixed-methodology approach combining primary interviews with neonatologists, biomedical engineers, supply chain executives, and regulatory affairs specialists. Quantitative data were collected through proprietary surveys distributed to over 150 NICUs across North America, Europe, and Asia-Pacific, supplemented by secondary sources such as industry white papers, peer-reviewed clinical studies, and public regulatory filings.
To ensure data validity and consistency, a triangulation framework was applied, reconciling insights from disparate sources and cross-referencing production capacity, clinical adoption rates, and device performance metrics. Market segmentation analyses were conducted using a bottom-up micro-model that maps end-user procurement behaviors to product specifications and application requirements.
Regulatory impact assessments were informed by a comprehensive review of Section 301 tariff notices, trade commission reports, and industry association commentaries. Regional market dynamics leveraged trade data, hospital expenditure databases, and adoption curve modelling. Qualitative thematic analysis of stakeholder interviews provided nuanced perspectives on innovation drivers, competitive strategies, and operational challenges.
This structured methodology underpins the credibility and actionable relevance of the findings presented in this executive summary, offering a transparent blueprint for replicability and future research extensions.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Neonatal Infant Care market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Neonatal Infant Care Market, by Product Type
- Neonatal Infant Care Market, by Therapeutic Area
- Neonatal Infant Care Market, by Distribution Channel
- Neonatal Infant Care Market, by Application
- Neonatal Infant Care Market, by End User
- Neonatal Infant Care Market, by Region
- Neonatal Infant Care Market, by Group
- Neonatal Infant Care Market, by Country
- United States Neonatal Infant Care Market
- China Neonatal Infant Care Market
- Competitive Landscape
- List of Figures [Total: 17]
- List of Tables [Total: 4293 ]
Concluding Reflections on Strategic Priorities and Collaborative Pathways to Advance Neonatal Infant Care Excellence and Sustainability
The neonatal infant care market stands at a pivotal juncture where clinical imperatives, technological advancements, and policy interventions converge to shape future trajectories. As tariff landscapes shift and competitive pressures mount, stakeholders must adopt agile strategies that balance risk mitigation with innovation acceleration. The era of siloed device deployment is giving way to integrated systems that not only deliver critical life-saving functionality but also generate actionable data insights.
Embracing interoperable platforms and modular device architectures will be key to unlocking value across product lifecycles, from design and validation to maintenance and upgrades. Collaboration among device manufacturers, healthcare providers, and digital ecosystem partners will catalyze the co-creation of solutions that address nuanced clinical needs while satisfying regulatory compliance and sustainability benchmarks.
By synthesizing segmentation, regional, and company-level insights, organizations can refine their strategic playbooks to capitalize on high-growth opportunities-such as home-based neonatal services, tele-NICU expansions, and service-based commercial models-while safeguarding continuity of care. The collective commitment to data-driven innovation, interdisciplinary research, and stakeholder engagement will ultimately define success across the neonatal infant care ecosystem.
Empowering Decision-Makers with a Clear Call-to-Action to Engage with Associate Director Ketan Rohom for In-Depth Neonatal Infant Care Market Insights
To explore the full breadth of the neonatal infant care market research report, including in-depth analyses of emerging technological trends, regulatory impacts such as the 2025 U.S. tariff adjustments, and granular segmentation and regional insights, please reach out directly to Ketan Rohom, Associate Director for Sales & Marketing at 360iResearch. Engaging with Ketan will provide you with tailored guidance on how the findings relate to your strategic priorities, access to proprietary data models, and a personalized demonstration of supplementary datasets and scenario analyses critical to your decision-making process.
Ketan Rohom can help arrange a confidential briefing to walk you through specific sections of the report most relevant to your organization’s objectives, as well as discuss volume license options, custom research add-ons, or executive workshops designed around these insights. Secure your competitive advantage in the rapidly evolving neonatal infant care landscape by scheduling your consultation today and unlocking the full potential of this comprehensive market intelligence.

- How big is the Neonatal Infant Care Market?
- What is the Neonatal Infant Care Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




