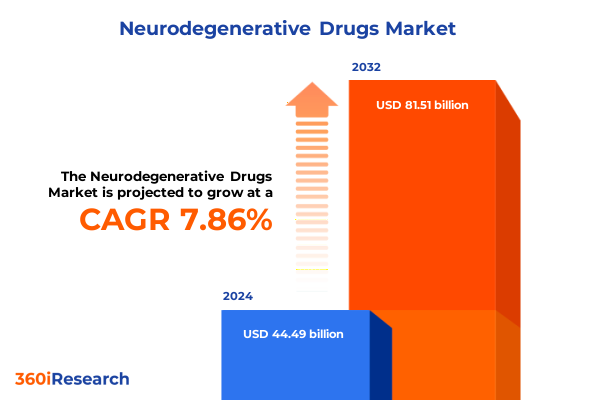
The Neurodegenerative Drugs Market size was estimated at USD 47.79 billion in 2025 and expected to reach USD 51.39 billion in 2026, at a CAGR of 7.92% to reach USD 81.51 billion by 2032.
Unraveling the Current Paradigm in Neurodegenerative Drug Development and Market Dynamics That Are Shaping the Next Wave of Therapeutic Innovations
According to the latest Alzheimer’s Association report, more than 7.2 million Americans aged 65 and older are living with Alzheimer’s disease, highlighting a significant rise in prevalence that places mounting pressures on healthcare systems, caregivers, and economic resources. This demographic shift underscores the urgent need for innovative therapies capable of addressing the multifaceted pathophysiology of neurodegenerative disorders.
Amid this growing burden, regulatory bodies have begun approving disease-modifying treatments that target core pathological mechanisms. The conversion of lecanemab’s accelerated approval to traditional approval by the U.S. Food and Drug Administration represents the first instance of an amyloid-beta–targeting monoclonal antibody receiving full approval, reshaping expectations for future therapeutic interventions.
Emerging Scientific Breakthroughs and Strategic Collaborations Propelling a Transformative Shift in Neurodegenerative Treatment Modalities
Immunotherapeutic candidates targeting tau pathology have recently taken center stage, as exemplified by AC Immune’s progression of its anti-pTau active immunotherapy into a registration-enabling Phase 2b study and Johnson & Johnson’s Fast Track designations for posdinemab, both of which aim to intercept disease progression at the molecular level. These advances illustrate a powerful convergence of immunology and precision medicine, offering hope for interventions that could arrest cognitive decline before symptom onset.
Simultaneously, the integration of artificial intelligence and proteomics is driving a transformative shift in discovery methodologies. The Global Neurodegeneration Proteomics Consortium’s landmark proteomic dataset, combined with AI-powered knowledge graphs and quantum computing–enhanced platforms, is accelerating target identification, biomarker validation, and candidate optimization. This hybrid computational approach marks an inflection point where data-driven insights and novel algorithmic models converge to shorten development timelines and expand the therapeutic horizon for neurodegenerative diseases.
Evaluating the Far-Reaching Effects of 2025 U.S. Tariff Policies on Global Supply Chains and Access to Neurodegenerative Therapies
Beginning in April 2025, the imposition of a 10% global tariff on nearly all imports into the United States has introduced new cost structures for active pharmaceutical ingredients, medical devices, and diagnostic tools, forcing pharmaceutical companies to reevaluate their sourcing strategies and supply chain configurations. These measures, intended to strengthen domestic manufacturing, have had the unintended consequence of elevating production expenses for both generics and branded therapies, potentially leading to constrained access for patients and strategic pivots toward alternative suppliers.
Moreover, reciprocal tariffs of up to 245% on Chinese APIs and 20–25% duties on intermediate drug substances have disproportionately affected generics manufacturers, disrupting the supply of essential medications and heightening concerns about shortages and quality variability. Industry stakeholders are exploring reshoring initiatives and diversified global networks to mitigate these risks, yet the path forward demands careful balancing of cost containment, regulatory compliance, and therapeutic continuity.
Deciphering Multifaceted Segmentation Insights to Illuminate Niche Opportunities Within the Neurodegenerative Drug Landscape Across Therapeutic and Patient Dimensions
When assessing the neurodegenerative drug landscape through the lens of product type, the interplay between branded innovations and cost-efficient generics shapes commercialization strategies and formulary inclusion. Classifying therapies by mechanism reveals distinct pathways: cholinesterase inhibitors such as donepezil, pyridostigmine, and rivastigmine maintain their symptomatic relevance, while decarboxylase inhibitors like benserazide, carbidopa, and methyldopa continue to play supportive roles in restoring neurotransmitter balance. Dopamine agonists including apomorphine, cabergoline, pramipexole, and rotigotine address motor symptoms, even as immunomodulators gain ground as next-generation candidates.
Mode of administration further differentiates product access and patient adherence, with injectable formulations offering rapid bioavailability, oral therapies providing convenience, and transdermal patches enabling sustained release. On the molecular front, the distinction between ion channel modulators and synaptic modulators-especially the subdivision of ion channel agents into potassium and sodium channel targets-underscores nuanced pharmacodynamic profiles. Therapeutic approaches range from neuroprotection using anti-inflammatory agents and antioxidants to preventive regimens and symptomatic treatments, reflecting a holistic continuum of care.
Indication-specific segmentation spans Alzheimer’s disease, amyotrophic lateral sclerosis, multiple sclerosis in its primary progressive and relapsing–remitting forms, and Parkinson’s disease, ensuring targeted research and clinical trial design. Patient demographics guide market models, differentiating adult, geriatric, and pediatric populations, while sales channel insights illuminate the balance between hospital and retail pharmacies, along with burgeoning online platforms. Finally, end-user analysis highlights the roles of clinics, contract research organizations, hospitals, and research institutes in driving adoption, clinical development, and post-market surveillance.
This comprehensive research report categorizes the Neurodegenerative Drugs market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Type
- Drug Class
- Mode Of Administration
- Mechanism Of Action
- Therapeutic Approach
- Indication
- Patient Demographics
- Sales Channel
- End User
Unearthing Critical Regional Disparities and Growth Drivers Shaping the Neurodegenerative Drug Ecosystem Across Americas, EMEA, and Asia-Pacific
In the Americas, a robust innovation ecosystem anchored by the United States fosters rapid adoption of novel therapies, underpinned by significant R&D investment, streamlined regulatory pathways, and an established reimbursement framework. Accelerated approvals and robust clinical trial networks enable early market entry for breakthrough agents, while private and public payers navigate evolving value-based pricing models to manage long-term care costs and patient access.
Europe, the Middle East, and Africa present a mosaic of regulatory and reimbursement environments. The European Commission’s recent authorization of lecanemab as the first disease-modifying therapy in the EU and the divergent decisions by health technology assessment bodies highlight a complex landscape where cost-effectiveness thresholds, budget impact analyses, and national healthcare priorities dictate patient access timelines. Emerging markets in the Middle East and select African nations are beginning to align with global standards, though infrastructure and funding gaps persist.
Across Asia-Pacific, governments and commercial stakeholders are intensifying efforts to localize manufacturing, streamline approvals, and expand public insurance coverage for high-cost biologics. Japan’s priority designation and rapid launch of lecanemab under its National Health Insurance system exemplify proactive strategies, while regional hubs in South Korea, China, and Australia are investing in clinical research and capacity building to meet the rising demand for neurodegenerative treatments.
This comprehensive research report examines key regions that drive the evolution of the Neurodegenerative Drugs market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Profiling Leading Industry Players and Their Strategic Initiatives Driving Innovation and Competition in Neurodegenerative Therapeutic Development
Biogen and Eisai have set the standard for collaborative development, with their joint commercialization of lecanemab and recent transition from accelerated to traditional approval demonstrating the power of strategic alliances and rigorous confirmatory trials in achieving regulatory milestones. Their dynamic pipeline and supplemental dosing applications further emphasize a lifecycle management approach centered on patient outcomes and real-world evidence integration.
Eli Lilly’s donanemab program exemplifies the potential of precision-targeted amyloid therapies, as evidenced by compelling Phase 3 trial data and regulatory submissions in the United States, Europe, and Asia-Pacific. Investments in biomarker-driven trial designs and risk mitigation strategies for amyloid-related imaging abnormalities reinforce the company’s commitment to balancing efficacy with safety in disease-modifying treatment frameworks.
AC Immune’s partnership with Janssen highlights the growing trend of licensing and milestone-driven collaborations for next-generation anti-tau immunotherapies. Meanwhile, emerging entrants specializing in small molecule and gene-based modalities are expanding competitive dynamics, underscoring the importance of diversified portfolios that address multiple facets of neurodegeneration.
This comprehensive research report delivers an in-depth overview of the principal market players in the Neurodegenerative Drugs market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Abbott Laboratories
- AbbVie Inc.
- ACADIA Pharmaceuticals Inc.
- Alpha Cognition Inc.
- Amneal Pharmaceuticals, Inc.
- Aquinnah Pharmaceuticals Inc.
- Asceneuron SA
- AstraZeneca PLC
- AZTherapies, Inc.
- Bausch Health Companies Inc.
- Biogen Inc.
- Boehringer Ingelheim International GmbH
- Denali Therapeutics Inc.
- Eli Lilly and Company
- GlaxoSmithKline PLC
- H. Lundbeck A/S
- Johnson & Johnson Services, Inc.
- Kyowa Kirin International plc
- Lupin Limited
- Merck & Co., Inc.
- Novartis AG
- Orion Corporation by Smiths Group plc
- Pfizer, Inc.
- Sanofi SA
- Takeda Pharmaceutical Company Limited
- Theravance Biopharma
- UCB S.A.
Actionable Strategic Recommendations for Industry Leaders to Navigate Challenges and Capitalize on Opportunities in Neurodegenerative Drug Development
Stakeholders should prioritize the establishment of flexible manufacturing platforms and strategic sourcing partnerships to mitigate tariff-induced supply chain risks and ensure continuity for critical therapies. Embracing modular production and dual-source procurement can safeguard against disruptions while preserving cost efficiencies.
Investing in adaptive clinical trial designs that leverage digital biomarkers, remote monitoring, and decentralized approaches will accelerate recruitment, reduce patient burden, and enhance data quality. Cross-sector collaborations with technology providers can enable real-time analytics and personalized dosing strategies, fostering more efficient pathways from concept to approval.
Payers and policymakers should collaborate to develop innovative reimbursement models, such as outcomes-based contracts and annuity payments, to align incentives across the product lifecycle. Early engagement with health technology assessment bodies, combined with robust health economics research, will expedite market access and ensure equitable patient coverage.
Finally, companies must deepen patient and caregiver engagement by co-creating educational programs, support services, and adherence tools. By integrating patient-reported outcomes into development strategies and post-market studies, industry leaders can refine product value propositions and strengthen market differentiation.
Rigorous Mixed-Method Research Methodology Underpinning the Comprehensive Analysis of Neurodegenerative Drug Development and Market Dynamics
This analysis integrates primary research through structured interviews with key opinion leaders, including neurologists, pharmacologists, and supply chain executives, complemented by an extensive survey of industry stakeholders. Secondary research encompassed a review of peer-reviewed literature, regulatory filings, clinical trial registries, and publicly available corporate disclosures.
Data triangulation methods were employed to reconcile insights from diverse sources, ensuring reliability and minimizing bias. A multi-dimensional framework underpinned segmentation analysis, considering factors such as therapeutic mechanism, patient demographics, and channel dynamics. Regional assessments incorporated regulatory timelines, reimbursement landscapes, and local market intelligence.
Quality control protocols included iterative expert validation workshops and cross-referencing of quantitative datasets with qualitative findings. Ethical guidelines and confidentiality standards were strictly adhered to throughout the research process, ensuring the integrity and transparency of the resulting insights.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Neurodegenerative Drugs market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Neurodegenerative Drugs Market, by Type
- Neurodegenerative Drugs Market, by Drug Class
- Neurodegenerative Drugs Market, by Mode Of Administration
- Neurodegenerative Drugs Market, by Mechanism Of Action
- Neurodegenerative Drugs Market, by Therapeutic Approach
- Neurodegenerative Drugs Market, by Indication
- Neurodegenerative Drugs Market, by Patient Demographics
- Neurodegenerative Drugs Market, by Sales Channel
- Neurodegenerative Drugs Market, by End User
- Neurodegenerative Drugs Market, by Region
- Neurodegenerative Drugs Market, by Group
- Neurodegenerative Drugs Market, by Country
- United States Neurodegenerative Drugs Market
- China Neurodegenerative Drugs Market
- Competitive Landscape
- List of Figures [Total: 21]
- List of Tables [Total: 2703 ]
Synthesizing the Journey of Neurodegenerative Drug Innovation and Market Transformation Toward an Era of Precision Therapeutics and Patient-Centric Care
The evolution of neurodegenerative drug development is marked by groundbreaking scientific innovations, shifting regulatory paradigms, and complex global dynamics. From the emergence of disease-modifying immunotherapies targeting amyloid and tau to the integration of AI-driven discovery and supply chain resilience strategies, the ecosystem is undergoing a transformational phase.
Stakeholders who embrace collaborative partnerships, data-centric development models, and patient-focused value frameworks will be best positioned to navigate the intricacies of this rapidly advancing landscape. As therapies move from symptomatic management toward true modification of disease progression, the promise of improving quality of life for millions moves ever closer to reality.
Engage With Associate Director Ketan Rohom to Secure Exclusive Insights and Access the Full Market Research Report on Neurodegenerative Drugs Today
If you’re ready to bring unparalleled depth and clarity to your strategic planning and decision-making, reach out to Ketan Rohom, Associate Director, Sales & Marketing at 360iResearch. Ketan’s expertise and personalized guidance will ensure you secure the comprehensive market research report that delivers actionable insights on neurodegenerative drug development, regulatory shifts, and competitive landscapes. Connect with Ketan today to explore tailored solutions and elevate your organization’s market positioning with the rigor and precision that only a full report can provide

- How big is the Neurodegenerative Drugs Market?
- What is the Neurodegenerative Drugs Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




