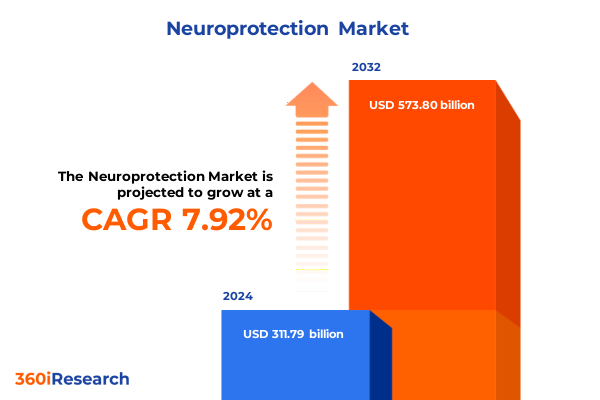
The Neuroprotection Market size was estimated at USD 336.31 billion in 2025 and expected to reach USD 359.74 billion in 2026, at a CAGR of 7.93% to reach USD 573.80 billion by 2032.
Understanding the Critical Role of Neuroprotection and the Evolving Imperative to Safeguard Neurological Health in Modern Therapeutics
Neuroprotection has emerged as a cornerstone of modern therapeutic development, reflecting an urgent need to preserve and restore neural function against a backdrop of rising neurological disorders. Conditions such as Alzheimer’s disease, Parkinson’s disease, and stroke continue to challenge healthcare systems globally, fueling an imperative for advanced interventions that go beyond symptom management. As our understanding of neuronal resilience deepens, scientists have begun to explore multifaceted strategies that integrate biologics, small molecules, gene therapies, and complementary approaches to safeguard and regenerate nervous tissue.
In this context, the neuroprotection market is distinguished by its convergence of cutting-edge research and clinical urgency. Innovations in monoclonal antibodies and neurotrophic factors stand alongside emerging gene-editing platforms and nutraceutical regimens, each promising to modify disease trajectories. This mosaic of modalities underscores a transformative era in which preventing cell death and fostering synaptic maintenance are as critical as targeting pathological hallmarks. Stakeholders across industry, academia, and patient advocacy groups are collaborating more closely than ever to translate preclinical breakthroughs into viable treatment paradigms.
Exploring the Revolutionary Advances and Transformative Shifts Redefining Neuroprotection Strategies Across the Biomedical Landscape
The neuroprotection ecosystem is undergoing a paradigm shift fueled by breakthroughs in molecular biology, precision medicine, and digital therapeutics. Gene therapy platforms leveraging viral vectors have achieved milestone successes in targeted delivery of neuroprotective genes, paving the way for sustained expression of critical growth factors and enzymes. Meanwhile, advances in monoclonal antibody engineering have yielded agents capable of crossing the blood–brain barrier with improved pharmacokinetics, opening doors to disease-modifying interventions in chronic neurodegenerative states.
Concurrently, the integration of artificial intelligence and high-throughput screening is accelerating the identification of novel candidate molecules that modulate neuroinflammatory pathways. Antioxidant therapies have evolved beyond classical vitamins to encompass tailored flavonoid derivatives with enhanced bioavailability and blood–brain barrier penetration. Additionally, digital biomarkers and remote monitoring solutions are redefining clinical trial design, allowing for continuous patient assessment and adaptive protocols. These technological and methodological innovations collectively represent a transformative trajectory that is redefining what is possible in neuroprotective R&D and care delivery.
Analyzing How Recent United States Tariffs Since 2025 Have Reshaped Supply Chains, Cost Structures and Innovation in Neuroprotection
Since the introduction of new tariff measures in early 2025, the United States has enacted levies on a range of imported pharmaceutical ingredients, laboratory reagents, and specialized medical equipment integral to neuroprotective research and manufacturing. This policy shift has introduced a new layer of complexity for organizations reliant on global supply chains, driving up input costs and elongating lead times for critical materials. Companies have responded by reassessing procurement strategies, exploring domestic sourcing partnerships, and in some cases, reshoring key manufacturing steps to mitigate exposure to import duties.
These cumulative tariffs have also stimulated investment in regional hub development, as stakeholders seek to establish resilient networks for biologics production and advanced API synthesis. While higher operational expenses have pressured margins in the short term, the imperative to localize supply chains has spurred innovation in process optimization and quality control. In parallel, regulatory agencies have offered guidance on streamlined approval pathways for domestically produced batches, encouraging efficient scale-up of neuroprotective compounds. As a result, the industry finds itself balancing cost containment with the pursuit of agile, secure sourcing and manufacturing strategies.
Unveiling Critical Market Segmentation Insights That Illuminate Product Types, Mechanisms, End Users, Indications, and Distribution Channels
Market segmentation in neuroprotection reveals distinct product categories, each characterized by unique developmental pathways and therapeutic rationales. Based on product type, the landscape spans Biologics, Drugs, Gene Therapy, Nutraceuticals, and Stem Cell Therapy. Within the biologics category, monoclonal antibody biologics harness targeted immune modulation, while neurotrophic factor biologics focus on sustaining neuronal viability and plasticity. The drugs segment divides into peptide drugs, offering high specificity with favorable safety profiles, and small molecule drugs, which provide oral bioavailability and blood–brain barrier penetration. Gene therapy initiatives employ refined viral and nonviral vectors to deliver corrective genetic payloads, whereas nutraceuticals encompass herbal extract nutraceuticals, mineral nutraceuticals, and vitamin nutraceuticals, reflecting patient-driven demand for preventive and adjunctive care. Stem cell therapy research continues to advance, with pluripotent and progenitor cell populations under investigation for their repair potential.
When examining mechanisms of action, anti-inflammatory agents take center stage, including cytokine inhibitor anti-inflammatory agents, NSAID anti-inflammatory agents, and steroid anti-inflammatory agents that mitigate deleterious neuroinflammation. Antioxidants such as flavonoid antioxidants, vitamin C antioxidants, and vitamin E antioxidants neutralize free radicals, preserving neuronal microenvironments. Calcium channel blockers like flunarizine and nimodipine control ion flux to prevent excitotoxic damage, while glutamate inhibitors and NMDA antagonists-exemplified by ketamine and memantine-attenuate glutamatergic overstimulation. Neurotrophic factors amplify intrinsic survival signals, counterbalancing degenerative cascades.
End users comprise clinics, home healthcare providers, hospitals, and research institutes, each demanding tailored delivery formats and support services. Clinical environments emphasize acute intervention efficacy, home healthcare solutions prioritize ease of administration and patient adherence, and research institutes drive preclinical and translational discovery. Therapeutic indications extend across Alzheimer’s disease-where interventions target early, moderate, and severe stages-through multiple sclerosis subtypes such as relapsing remitting and secondary progressive forms, as well as Parkinson’s disease with postural instability and tremor dominant phenotypes. Acute care strategies address hemorrhagic stroke, ischemic stroke, spinal cord injury, and traumatic brain injury. Distribution channels span hospital pharmacies with institutional bulk handling capabilities, online pharmacies offering direct-to-patient convenience, and retail pharmacies providing local community access, reflecting a multidimensional approach to delivering neuroprotective therapies.
This comprehensive research report categorizes the Neuroprotection market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Product Type
- Mechanism Of Action
- Indication
- Distribution Channel
- End User
Decoding Regional Dynamics in the Neuroprotection Market to Reveal Opportunities and Challenges Across Americas, EMEA, and Asia-Pacific
Regional dynamics in the neuroprotection market are shaped by diverging healthcare infrastructures, regulatory landscapes, and funding priorities. In the Americas, robust venture capital ecosystems and government incentives have accelerated early-stage biotech ventures, while established pharmaceutical companies advance late-stage clinical programs. Public–private partnerships in this region are catalyzing translational research, fueling a pipeline rich with novel biologics and gene therapies aimed at chronic neurodegenerative conditions.
Europe, Middle East & Africa display heterogeneity in uptake and reimbursement policies, where centralized European regulatory approvals coexist with country-specific health technology assessment frameworks. Collaborative networks across academic centers in Western Europe have fostered multicenter clinical trials, whereas emerging markets in the Middle East and Africa prioritize capacity building and increasing access to foundational neuroprotective care.
Asia-Pacific stands out for its combination of large patient populations, evolving reimbursement environments, and significant manufacturing capacity. Innovation hubs in East Asia drive high-volume production of generic neuroprotective agents, while biotech clusters in Southeast Asia focus on next-generation modalities such as stem cell and gene therapies. In parallel, regulatory authorities across the region are harmonizing guidelines to support accelerated approval pathways, reflecting a strategic commitment to addressing the rising burden of neurodegenerative diseases.
This comprehensive research report examines key regions that drive the evolution of the Neuroprotection market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Profiling Leading Industry Players and Their Strategic Initiatives Driving Innovation and Competitive Differentiation in Neuroprotection
Key industry players spanning multinational pharmaceutical corporations, emerging biotech firms, and specialized technology providers are reshaping the neuroprotection competitive landscape. Large-cap organizations have leveraged extensive R&D infrastructures to advance high-profile clinical candidates targeting Alzheimer’s and Parkinson’s diseases, often through strategic acquisitions and global licensing deals. Their deep pipelines benefit from cross-disciplinary platforms that integrate antibody engineering, viral vector optimization, and advanced formulation science.
Simultaneously, innovative biotech startups are carving out niche positions by focusing on precision therapies for rare neurodegenerative subtypes, utilizing proprietary gene-editing technologies and artificial intelligence–driven compound discovery. Collaboration between these agile companies and academic research institutes has accelerated proof-of-concept studies, while strategic partnerships with drug delivery specialists aim to overcome blood–brain barrier challenges.
Technology providers have contributed to the ecosystem through the development of digital monitoring tools and remote assessment platforms, enabling real-time patient data capture and adaptive trial designs. Collectively, these players are driving competitive differentiation through a combination of platform innovation, targeted M&A, and integrated solution offerings that span discovery to delivery.
This comprehensive research report delivers an in-depth overview of the principal market players in the Neuroprotection market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- AC Immune SA
- Alector, Inc.
- Amgen Inc.
- AstraZeneca PLC
- Axsome Therapeutics, Inc.
- BioArctic AB
- Biogen Inc.
- Cassava Sciences, Inc.
- Denali Therapeutics, Inc.
- Eisai Co., Ltd.
- Eli Lilly and Company
- F. Hoffmann-La Roche Ltd
- Ionis Pharmaceuticals, Inc.
- Neurocrine Biosciences, Inc.
- Novartis AG
- Pfizer Inc.
- Takeda Pharmaceutical Company Limited
- UCB S.A.
- Vivoryon Therapeutics N.V.
- Voyager Therapeutics, Inc.
Presenting Strategic Recommendations to Guide Industry Leaders in Navigating Market Turbulence and Capitalizing on Emerging Neuroprotection Trends
Industry leaders must adopt integrated strategies that balance rapid innovation with robust risk management in an environment of regulatory complexity and supply chain volatility. First, fostering cross-sector collaborations between pharmaceutical companies, academic laboratories, and technology vendors will accelerate the translation of preclinical discoveries into clinical realities. Establishing joint development agreements and shared infrastructure can optimize resource allocation and reduce time to market.
Second, rethinking supply chain resilience in light of recent tariff-induced disruptions is essential. Companies should explore dual sourcing models that leverage both domestic manufacturing hubs and strategic international partnerships. Investing in modular, flexible manufacturing facilities can enable rapid scale-up of biologics and advanced therapies while mitigating import dependency.
Third, aligning product development with emerging reimbursement frameworks will be critical. Engaging early with payers and health technology assessment bodies to demonstrate value through real-world evidence and patient-reported outcomes can secure favorable coverage. Moreover, integrating digital health solutions for monitoring and adherence will enhance value propositions and support differentiated market access strategies.
Finally, prioritizing patient-centric innovation through co-design initiatives and expanded access programs will strengthen brand loyalty and foster long-term engagement. By keeping end-user needs at the forefront, industry leaders can develop neuroprotective solutions that resonate with clinicians, caregivers, and patients alike, ensuring sustainable growth and impact.
Detailing Robust Research Methodologies, Data Sources, and Analytical Frameworks That Underpin the Neuroprotection Market Intelligence
This analysis leverages a dual-method research framework combining extensive secondary research with targeted primary investigations. Secondary data were collected from peer-reviewed journals, clinical trial registries, patent databases, regulatory filings, and specialist industry publications, ensuring a comprehensive understanding of technology platforms, regulatory pathways, and competitive activity. Complementary primary research involved structured interviews with key opinion leaders, including neurologists, pharmacologists, and R&D executives, to validate market dynamics and capture nuanced insights on therapeutic adoption and clinical unmet needs.
Quantitative data were synthesized through rigorous data triangulation techniques, cross-referencing supplier shipment volumes, pipeline disclosures, and conference proceedings to ensure accuracy. Qualitative inputs were analyzed using thematic coding frameworks to distill critical trends in innovation ecosystems and stakeholder priorities. A detailed segmentation model was applied across product types, mechanisms of action, end-user categories, indications, and distribution channels, enabling granular interpretation of market drivers and barriers. Finally, the findings were subjected to multiple validation rounds with external consultants and industry veterans, reinforcing the robustness of conclusions and supporting actionable recommendations.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Neuroprotection market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Neuroprotection Market, by Product Type
- Neuroprotection Market, by Mechanism Of Action
- Neuroprotection Market, by Indication
- Neuroprotection Market, by Distribution Channel
- Neuroprotection Market, by End User
- Neuroprotection Market, by Region
- Neuroprotection Market, by Group
- Neuroprotection Market, by Country
- United States Neuroprotection Market
- China Neuroprotection Market
- Competitive Landscape
- List of Figures [Total: 17]
- List of Tables [Total: 2703 ]
Synthesizing Core Findings and Strategic Implications to Provide a Forward-Looking Perspective on Neuroprotection Market Evolution
The evolving neuroprotection landscape reflects a confluence of scientific breakthroughs, shifting policy environments, and strategic supply chain adaptations. The integration of biologic platforms with gene therapy and advanced small molecule approaches underscores the sector’s innovation potential, while recent tariff measures have galvanized efforts to localize manufacturing and diversify sourcing strategies. Segmentation insights illuminate the multifaceted nature of product development, regulatory alignment, and delivery mechanisms that collectively define competitive advantage.
Regionally, the Americas, EMEA, and Asia-Pacific offer distinct growth corridors shaped by capital flows, regulatory harmonization, and manufacturing capabilities. Industry incumbents and nimble biotech entrants are leveraging these dynamics through targeted collaborations, digital health integration, and patient-centric program designs. Amid these trends, leading organizations are advised to maintain agility, invest in robust cross-sector partnerships, and engage proactively with payers to secure sustainable market access.
In conclusion, the neuroprotection market stands at a pivotal crossroads. Organizations that harness integrated R&D platforms, diversify supply chains, and emphasize real-world value demonstration will be best positioned to deliver on the promise of novel therapies, address unmet clinical needs, and achieve long-term commercial success.
Connect with Ketan Rohom to Unlock Comprehensive Neuroprotection Market Research Insights and Empower Your Strategic Decision-Making
If you are ready to leverage deep insights into the current and emerging neuroprotection landscape, connect directly with Ketan Rohom, Associate Director, Sales & Marketing at 360iResearch. His expertise in guiding organizations through complex market dynamics will help you tailor strategies that align with the latest therapeutic innovations, regulatory developments, and competitive movements. Engage Ketan today to explore customized data packages, detailed segment analyses, and privileged executive briefings that will position your organization for success in an increasingly competitive and rapidly evolving neuroprotection arena.

- How big is the Neuroprotection Market?
- What is the Neuroprotection Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




