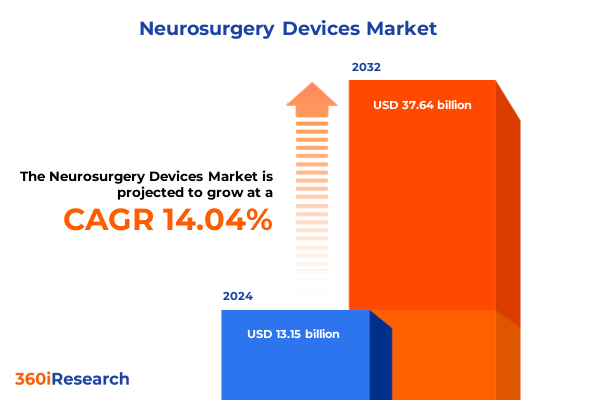
The Neurosurgery Devices Market size was estimated at USD 14.91 billion in 2025 and expected to reach USD 16.92 billion in 2026, at a CAGR of 14.13% to reach USD 37.64 billion by 2032.
Exploring the Evolution and Significance of Neurosurgery Devices in Modern Healthcare Landscapes with an Emphasis on Innovation and Patient Outcomes
The neurosurgery devices sector stands at the forefront of modern medical innovation, addressing intricate neurological disorders with unprecedented precision and efficacy. Over the past decade, the advent of high-resolution imaging and robotic-assisted interventions has redefined surgical standards, enabling clinicians to navigate complex brain and spinal procedures with reduced invasiveness and enhanced patient safety. Simultaneously, the integration of artificial intelligence-driven planning tools and augmented reality navigation systems has bolstered intraoperative decision-making, ensuring optimal placement of instruments and implants while minimizing the risk of collateral tissue damage.
Against this backdrop of technological evolution, neurosurgery devices have transcended their role as mere surgical tools to become critical components of comprehensive treatment ecosystems. From biologics that foster bone regeneration in spinal fusion to neurostimulators that modulate brain circuits in movement disorders, the spectrum of products addresses diverse therapeutic needs. Understanding the interplay among these innovations, alongside the regulatory and reimbursement environments, is essential for stakeholders aiming to capitalize on emerging opportunities and navigate potential challenges.
Identifying Revolutionary Shifts Reshaping Neurosurgery Device Development and Clinical Practice toward Minimally Invasive Techniques and Digital Integration
In recent years, the neurosurgery landscape has undergone transformative shifts as clinicians and device developers converge on minimally invasive techniques and digital integration. Augmented reality-based neuronavigation now allows surgeons to superimpose three-dimensional anatomical maps directly onto the operative field, improving P lacement accuracy and reducing operating times by delivering critical spatial cues in real time. Concurrently, robotic-assisted platforms have evolved from rigid mechanical supports to intelligent systems capable of adaptive feedback, merging human dexterity with machine precision to achieve submillimeter accuracy in trajectories for deep brain stimulation and spinal screw placement.
Moreover, the rise of artificial intelligence has introduced predictive analytics and intraoperative decision support into neurosurgery. Machine learning models analyze preoperative imaging and historical case data to recommend optimal surgical pathways and anticipate potential complications, enhancing both safety and efficiency. As these digital technologies mature, the boundaries between surgical planning, execution, and postoperative monitoring continue to blur, signaling a new era in which real-time data fusion and interconnected devices drive patient-centered outcomes.
Assessing the Multifaceted Consequences of Recent United States Tariff Measures on Neurosurgery Device Supply Chains and Production Costs in 2025
The imposition of new United States tariffs in 2025 has introduced significant headwinds across neurosurgery device supply chains and production cost structures. Import duties ranging from 10 percent on general medical equipment to as high as 145 percent on key high-end components have compelled manufacturers to reevaluate sourcing strategies and operational footprints. Tariffs on precision sensors and motors used in robotic platforms have increased manufacturing costs and pressured profit margins, prompting both established device makers and smaller innovators to intensify efforts in tariff exclusion petitions and legislative advocacy.
Simultaneously, prominent medtech firms are accelerating investments in domestic production capabilities to mitigate the impact of trade levies. Leading companies have announced expansions of U.S. manufacturing sites in Georgia and California, while others optimize existing operations to comply with regional trade agreements and reduce exposure to duties. Despite these mitigation steps, hospitals and healthcare facilities may ultimately bear increased equipment expenditures, potentially affecting capital procurement cycles and delaying the adoption of next-generation neurosurgical tools.
Uncovering Critical Insights from Product, End User, Application, and Procedure Segmentation to Illuminate Neurosurgery Device Market Dynamics and Demand Drivers
A nuanced understanding of market segmentation reveals the drivers behind adoption patterns and investment priorities in neurosurgery devices. Product Type segmentation highlights six core categories: biologics engineered for bone grafts and growth factors; endoscopes, both flexible and rigid, enabling high-definition visualization; implantable devices, spanning cranial plates, dural substitutes, fusion cages and pedicle screws; precision instruments such as drills, retractors, and ultrasonic aspirators; navigation systems leveraging electromagnetic and optical modalities; and neurostimulators, including deep brain and vagus nerve stimulators. Each segment addresses specific procedural challenges, from regenerative therapies supporting spinal fusion to neurostimulation solutions treating movement disorders and epilepsy.
Similarly, End User segmentation differentiates the purchasing landscapes of ambulatory surgical centers, hospitals, research institutes, and specialty clinics, each driven by unique workflow requirements and procedural volumes. Application-focused grouping underscores treatment areas such as brain tumors, epilepsy, hydrocephalus, movement disorders, pain management, and spinal cord disorders, reflecting the broad clinical reach of these devices. Finally, Procedure categorization distinguishes among endoscopic surgery, laser therapy, minimally invasive surgery, and open surgery, illustrating how device selection aligns with surgical complexity and patient recovery considerations. Together, these segmentation insights illuminate the multifaceted nature of demand and guide manufacturers in tailoring product development, marketing, and support strategies.
This comprehensive research report categorizes the Neurosurgery Devices market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Product Type
- Procedure
- End User
- Application
Comparative Analysis of Regional Variations in Neurosurgery Device Adoption across the Americas, Europe Middle East Africa, and Asia-Pacific Markets
Regional variations in neurosurgery device adoption underscore the importance of localized strategies and regulatory environments. In the Americas, established healthcare systems and robust reimbursement frameworks have fueled the uptake of advanced minimally invasive platforms and neurostimulation therapies, with hospitals and surgery centers at the forefront of integrating novel biologics and digital navigation solutions. Regulatory clarity and incentives for domestic manufacturing have also supported growth amid recent trade policy shifts.
Within Europe, the Middle East, and Africa, a blend of mature markets and emerging economies presents a heterogeneous landscape. Western European countries prioritize value-based procurement and rigorous clinical validation, driving demand for highly accurate navigation systems and implantable devices with strong safety profiles. In many Middle Eastern nations, investments in state-of-the-art medical infrastructure and cross-border partnerships accelerate access to cutting-edge neurosurgical technologies. Meanwhile, sub-Saharan Africa and parts of the Middle East are at earlier stages of market maturation, with demand concentrated in major hospital hubs and research institutions exploring targeted applications such as hydrocephalus treatment and neurostimulation interventions.
Asia-Pacific markets exhibit rapid expansion driven by healthcare infrastructure development, rising neurological disorder prevalence, and government initiatives to improve access to specialized care. Countries like Japan and South Korea lead in robotic and AR-assisted procedures, while China and India are scaling production capabilities and localizing manufacturing to meet domestic demand. Across the region, growing investments in training programs and academic collaborations support the diffusion of best practices and innovative device applications.
This comprehensive research report examines key regions that drive the evolution of the Neurosurgery Devices market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Profiling the Strategic Initiatives, Innovations, and Competitive Positioning of Leading Companies Driving Progress in the Neurosurgery Devices Sector
Leading companies in the neurosurgery devices sector have pursued diverse strategies to maintain competitiveness and foster innovation. Intuitive Surgical has emphasized supply chain resilience and margin management, relocating critical component production to Mexico and the United States while highlighting tariff mitigation in its financial communications. Boston Scientific and Abbott Laboratories have expanded U.S. manufacturing footprints, investing in new facilities in Georgia, Minnesota, Illinois, and Texas to support their portfolios of neurovascular, spine, and transfusion devices, thereby reducing exposure to import duties and strengthening delivery capabilities.
At the same time, Medtronic and Siemens Healthineers have bolstered their navigation and imaging systems through strategic acquisitions and R&D collaborations, integrating real-time imaging enhancements and AI-driven analytics to deliver comprehensive digital surgery platforms. Smaller innovators, including robotics startups and flexible endoscope developers, continue to carve niche positions by focusing on single-use technologies that address infection control and workflow efficiency. Across the competitive spectrum, ecosystem-centric approaches-bundling devices with training modules, procedural guidance, and data analytics services-underscore the shift toward value-added offerings that extend beyond hardware alone.
This comprehensive research report delivers an in-depth overview of the principal market players in the Neurosurgery Devices market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Abbott Laboratories
- B. Braun Melsungen AG
- Boston Scientific Corporation
- Globus Medical, Inc.
- Integra LifeSciences Holdings Corporation
- Johnson & Johnson
- Medtronic plc
- Penumbra, Inc.
- Smith & Nephew plc
- Stryker Corporation
- Terumo Corporation
- Zimmer Biomet Holdings, Inc.
Implementing Targeted Strategies for Industry Leaders to Navigate Regulatory Complexities, Enhance Innovation Pipelines, and Strengthen Global Market Presence
Industry leaders should prioritize the diversification of supply chains and localization of manufacturing to insulate operations from trade policy volatility. By establishing production capabilities within key markets and securing exemptions for critical components, organizations can enhance resilience and cost predictability. In parallel, accelerating investments in digital technologies-such as AI-powered planning tools, AR-guided navigation, and remote monitoring platforms-will position companies to meet evolving clinical demands for precision and efficiency.
To sustain competitive advantage, device manufacturers must also forge deeper partnerships with healthcare providers and academic institutions. Collaborative R&D initiatives can co-create next-generation biologics and neurostimulation solutions, while shared data ecosystems facilitate outcome tracking and continuous improvement. In addition, portfolio rationalization and modular product design will support rapid updates in response to regulatory changes and clinical feedback. By integrating these strategies within a proactive roadmap, industry participants can capitalize on near-term opportunities and future-proof their innovation pipelines.
Delineating the Comprehensive Research Framework Employed to Ensure Accuracy, Reliability, and Depth in Analyzing Neurosurgery Device Market Trends and Insights
This analysis was developed through a robust research framework combining secondary and primary data collection. Secondary research encompassed an extensive review of peer-reviewed journals, regulatory filings, patent databases, and credible news sources to identify technological trends, policy developments, and regional nuances. Primary research included in-depth interviews with neurosurgeons, medical device executives, and procurement specialists, providing nuanced perspectives on clinical needs, adoption barriers, and strategic priorities.
Data triangulation methods were employed to cross-validate findings and ensure consistency across multiple inputs. Quantitative and qualitative insights were synthesized to produce segmentation models that reflect real-world behaviors. Rigorous editorial reviews and expert consults further refined the analysis, ensuring that recommendations are both actionable and aligned with the latest industry dynamics.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Neurosurgery Devices market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Neurosurgery Devices Market, by Product Type
- Neurosurgery Devices Market, by Procedure
- Neurosurgery Devices Market, by End User
- Neurosurgery Devices Market, by Application
- Neurosurgery Devices Market, by Region
- Neurosurgery Devices Market, by Group
- Neurosurgery Devices Market, by Country
- United States Neurosurgery Devices Market
- China Neurosurgery Devices Market
- Competitive Landscape
- List of Figures [Total: 16]
- List of Tables [Total: 2067 ]
Summarizing Key Takeaways and the Strategic Importance of Neurosurgery Device Advancements for Stakeholders in a Rapidly Evolving Healthcare Environment
The convergence of minimally invasive technologies, digital integration, and strategic supply chain alignment is reshaping the neurosurgery devices landscape. From biologics enabling regenerative therapies to AI-enhanced navigation systems and advanced neurostimulation platforms, innovation continues to unlock improved patient outcomes while imposing new operational complexities. As tariffs and geopolitical factors influence cost structures and sourcing strategies, the need for agile responsiveness and localized capabilities has never been more critical.
Stakeholders across the value chain-manufacturers, healthcare providers, and policymakers-must collaborate to foster environments conducive to clinical validation and reimbursement support for emerging technologies. By balancing rigorous evidence generation with proactive investment in digital ecosystems and manufacturing infrastructure, the neurosurgery devices sector can sustain its momentum and deliver transformative care to patients worldwide.
Engage with Our Associate Director to Secure Expert Guidance and Access an In-Depth Neurosurgery Devices Market Research Report Tailored to Your Strategic Needs
I invite you to connect with Ketan Rohom, the Associate Director of Sales & Marketing, to secure a tailored briefing and exclusive access to our comprehensive market research report on neurosurgery devices. Drawing upon deep industry expertise and rigorous analysis, our report delivers actionable insights and strategic guidance essential for navigating the competitive landscape. Reach out to Ketan to schedule a consultation, learn how to leverage our findings for your organization’s growth, and obtain the full report customized to your objectives and market interests. Don’t miss this opportunity to empower your decision-making with unparalleled market intelligence.

- How big is the Neurosurgery Devices Market?
- What is the Neurosurgery Devices Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




