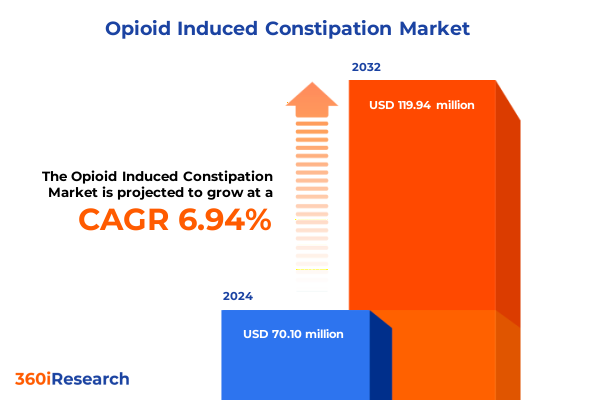
The Opioid Induced Constipation Market size was estimated at USD 75.03 million in 2025 and expected to reach USD 79.32 million in 2026, at a CAGR of 6.93% to reach USD 119.94 million by 2032.
Understanding the Multi-Dimensional Burden of Opioid Induced Constipation on Patients’ Quality of Life and the Advancements in Management Strategies
Opioid induced constipation represents a profound challenge for patients undergoing long-term opioid therapy, affecting both quality of life and treatment adherence. The condition arises when opioid molecules bind to peripheral mu-opioid receptors in the gastrointestinal tract, resulting in decreased motility, impaired secretion, and enhanced fluid absorption. Consequently, affected individuals often experience discomfort, bloating, and severe constipation that undermines overall therapeutic outcomes. As opioid prescriptions continue across oncologic, chronic non-cancer pain, and palliative care settings, there is mounting pressure on healthcare providers to integrate effective management strategies that do not compromise analgesic efficacy.
Moreover, recent shifts in prescribing practices, coupled with increased awareness of opioid adverse effects, have intensified the demand for dedicated therapeutics. Historically, over-the-counter laxatives and stool softeners provided first-line relief, yet their efficacy is often limited in severe cases, prompting a transition toward targeted pharmacologic interventions. In this context, peripherally acting mu-opioid receptor antagonists have emerged as a transformative class designed to counteract the constipating effects of opioids without altering central analgesia.
This executive summary delivers a concise overview of the current therapeutic landscape, explores significant shifts in treatment paradigms, evaluates tariff impacts on supply chains, and highlights key segmentation, regional, and competitive insights. By synthesizing rigorous research and industry expertise, this document establishes a foundation for informed decision-making to optimize patient care and commercial strategy in opioid induced constipation management.
Exploring How Novel Mechanisms of Action and Evolving Clinical Guidelines Are Reshaping Opioid Induced Constipation Treatment Paradigms
The therapeutic landscape for opioid induced constipation has undergone remarkable transformation as novel mechanisms of action and evolving clinical guidelines drive shifts in treatment paradigms. Traditional laxatives, once the dominant modality, now share prominence with combination therapies that integrate osmotic, stimulant, and stool softening agents to deliver more nuanced symptom relief. Concurrently, the approval and clinical uptake of peripherally acting mu-opioid receptor antagonists signal a decisive move toward targeted pharmacologic control of gastrointestinal motility without sacrificing systemic analgesia.
In parallel, guideline bodies have increasingly recommended stratified approaches, advocating for early intervention with receptor antagonists in patients at high risk for severe constipation or those who exhibit laxative-refractory symptoms. This strategic realignment underscores a broader emphasis on proactive symptom management rather than reactive rescue therapy. Furthermore, the integration of digital health tools and telemedicine platforms is enhancing patient monitoring, enabling real-time assessment of bowel function, and fostering improved adherence to prescribed regimens.
Collectively, these advancements not only expand the therapeutic toolkit but also reinforce the importance of individualized care pathways. As stakeholders adjust to this dynamic environment, they must navigate shifting reimbursement landscapes, regulatory complexities, and evolving prescriber preferences. Ultimately, the convergence of innovative drug classes, evidence-based guidelines, and patient-centric technologies is redefining the standard of care in opioid induced constipation management.
Assessing the Broad Repercussions of New United States Tariffs on the Opioid Induced Constipation Therapeutic Supply Chain and Pricing Dynamics in 2025
The introduction of new United States tariffs in early 2025 has introduced a complex variable into the supply chain dynamics for opioid induced constipation therapies. With key active pharmaceutical ingredients and excipients sourced from global suppliers, manufacturers now face increased import costs that reverberate through production budgets and ultimately influence portfolio pricing strategies. As a result, companies are reassessing sourcing models, evaluating nearshoring options, and investing in domestic manufacturing capabilities to mitigate exposure to fluctuating tariff schedules.
Moreover, the tariff impact extends beyond direct cost implications. Extended lead times for imported raw materials have strained inventory buffers, prompting organizations to adopt just-in-case stock management over traditional just-in-time processes. This shift entails greater working capital allocation but also ensures the continuity of supply for critical agents such as peripherally acting mu-opioid receptor antagonists. In effect, stakeholders must balance cost containment with robust contingency planning to preserve both margin integrity and patient access.
Looking forward, manufacturers are exploring strategic collaborations with contract development and manufacturing organizations to diversify production footprints and leverage scale efficiencies. Meanwhile, pricing negotiations with payers require a refined understanding of the cumulative tariff burden and its downstream effect on net reimbursement. By proactively addressing these challenges, industry participants can maintain competitive advantage and safeguard the delivery of essential opioid induced constipation treatments amid evolving trade policies.
Unveiling Critical Product Format and Administration Route Segmentation Insights to Inform Strategic Prioritization in Opioid Induced Constipation Management
A nuanced understanding of product and administration route segmentation offers strategic clarity in addressing diverse patient needs and optimizing resource allocation. Based on product type, the market is organized into combination therapies that synergistically blend agents to enhance efficacy, traditional laxatives which subdivide into osmotic, stimulant, and stool softeners tailored to different mechanisms, and peripherally acting mu-opioid receptor antagonists including Alvimopan, Methylnaltrexone, Naldemedine, and Naloxegol designed for targeted receptor blockade without central nervous system penetration.
Furthermore, the oral route yields distinct delivery formats, encompassing liquid formulations in the form of solutions and suspensions preferred for their ease of dose titration in vulnerable populations, alongside solid dosage options comprising capsules and tablets that offer convenience and dosing precision. Meanwhile, rectal administration remains vital, encompassing enemas and suppositories that provide rapid onset of action, particularly in acute or refractory scenarios where oral intake is compromised.
Collectively, these segmentation layers reveal critical insights. Combination therapies maintain prominence due to their broad mechanism coverage, while the oral solid segment captures significant volume given its established role in outpatient care. Emerging traction in peripherally acting agents underscores a shift toward targeted pharmacotherapies. In rectal administration, suppositories demonstrate sustained use in inpatient and long-term care settings. By aligning portfolio strategies with these nuanced segmentation dynamics, stakeholders can effectively target unmet needs, streamline distribution channels, and strengthen competitive positioning in opioid induced constipation management.
This comprehensive research report categorizes the Opioid Induced Constipation market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Product Type
- Route of Administration
- Distribution Channel
Comparative Regional Analysis Highlighting Distinct Adoption Patterns and Market Drivers Across Americas, Europe Middle East & Africa, and Asia Pacific
Regional variations in healthcare infrastructure, regulatory frameworks, and patient demographics drive distinct adoption trajectories in opioid induced constipation treatments. In the Americas, robust reimbursement mechanisms and an established culture of early intervention facilitate rapid uptake of advanced pharmacotherapies. Healthcare providers in this region increasingly integrate peripherally acting mu-opioid receptor antagonists as first- or second-line options, supported by extensive clinical education initiatives and well-defined prescribing guidelines.
Contrastively, Europe, Middle East & Africa presents a heterogeneous landscape where national formularies, price control policies, and varying levels of specialist access shape demand patterns. In Western Europe, centralized procurement and value-based purchasing are driving cost-effectiveness analyses, encouraging the adoption of receptor antagonists in high-risk cohorts. Simultaneously, markets in the Middle East and Africa often exhibit delayed access timelines, though expanding healthcare investment and growing awareness are creating opportunities for targeted therapies to address significant unmet need.
Moving to the Asia-Pacific region, rapid growth is underpinned by increasing healthcare expenditure, rising opioid usage for both cancer and chronic pain, and the development of local manufacturing capabilities. Regulatory authorities are progressively harmonizing approval processes, and formulary committees are beginning to recognize the clinical and economic value of tailored opioid induced constipation interventions. This confluence of factors positions the Asia-Pacific as a pivotal growth frontier for companies aiming to scale their portfolios globally.
This comprehensive research report examines key regions that drive the evolution of the Opioid Induced Constipation market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Profiling Leading Pharmaceutical and Biotech Innovators Driving Treatment Advances and Competitive Positioning in the Opioid Induced Constipation Arena
Leading pharmaceutical and biotech organizations continue to advance the opioid induced constipation space by leveraging differentiated portfolios and strategic alliances. AstraZeneca, through its Movantik product, capitalizes on naloxegol’s oral bioavailability and favorable safety profile, while Takeda’s Symproic harnesses the novel receptor affinity of naldemedine to address refractory cases. Pfizer’s Relistor maintains a strong presence in both subcutaneous and oral formulations of methylnaltrexone, broadening patient access through multiple administration options.
Alvimopan, marketed under Entereg by Covis, remains a key inpatient therapy due to its accelerated gastrointestinal transit benefits following surgical procedures. Beyond branded leaders, generic manufacturers are expanding their offerings of osmotic and stimulant laxatives, focusing on cost-efficient solutions to complement emerging prescription products. Partnerships between originators and specialty pharmacies further enhance patient support services, ensuring adherence monitoring and reimbursement assistance.
In addition, several mid-sized biotech firms are exploring next-generation receptor modulators and combination paradigms to capture niche segments. Licensing agreements and co-promotion deals are enabling market newcomers to leverage established distribution networks, while co-development projects are accelerating clinical pipelines. These evolving competitive dynamics underscore the importance of innovation, strategic collaboration, and differentiated value propositions in shaping the future of opioid induced constipation therapeutics.
This comprehensive research report delivers an in-depth overview of the principal market players in the Opioid Induced Constipation market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- AstraZeneca PLC
- Bausch Health Companies Inc.
- Cubist Pharmaceuticals LLC
- Daewoong Pharmaceutical Co., Ltd.
- Egalet Corporation
- GlaxoSmithKline PLC
- Ironwood Pharmaceuticals, Inc.
- Johnson & Johnson
- Merck & Co., Inc.
- Mundipharma International Limited
- Nektar Therapeutics
- Pfizer Inc.
- Pharmaceuticals Co., Ltd.
- Purdue Pharma L.P.
- Shionogi & Co., Ltd.
- Takeda Pharmaceutical Company Limited
- Trevena, Inc.
- Vertex Pharmaceuticals Incorporated
Strategic Imperatives and Tactical Approaches for Industry Leaders to Optimize Portfolios and Navigate Tariff Constraints in Opioid Induced Constipation
To thrive in the evolving opioid induced constipation landscape, industry leaders must adopt a multifaceted strategy that balances innovation, operational efficiency, and stakeholder engagement. First, optimizing portfolios involves prioritizing high-value assets such as peripherally acting mu-opioid receptor antagonists and developing differentiated formulations that enhance convenience and patient adherence. Concurrently, investing in patient support programs and digital adherence tools can amplify real-world outcomes and reinforce brand loyalty.
In response to tariff-induced cost pressures, companies should diversify their supply chains by forging strategic alliances with contract manufacturing organizations and exploring nearshoring opportunities. By doing so, they can mitigate price volatility and sustain uninterrupted delivery of critical therapies. Furthermore, proactive collaboration with payers and health technology assessment bodies will facilitate favorable reimbursement pathways, particularly when presenting pharmacoeconomic evidence that underscores long-term quality-of-life improvements.
Finally, geographic expansion requires a tailored approach that reflects local regulatory environments and market maturity. Leveraging regional partnerships, aligning with government health initiatives, and adapting pricing models to specific reimbursement frameworks will accelerate market entry and penetration. By integrating these tactical imperatives, stakeholders can navigate trade complexities, elevate treatment standards, and capture sustainable growth in opioid induced constipation management.
Illustrating a Mixed-Methods Research Framework of Expert Consultations and Secondary Analysis to Guarantee Rigorous Insights into Opioid Induced Constipation
This research integrates qualitative and quantitative methodologies to ensure robust, actionable insights into opioid induced constipation treatment dynamics. Primary data collection involved in-depth interviews with clinicians, key opinion leaders, and healthcare payers across major markets, capturing firsthand perspectives on clinical efficacy, patient adherence, and reimbursement challenges. Supplementary expert consultations with pharmacologists and supply chain specialists enriched the analysis of manufacturing and distribution intricacies.
Secondary research encompassed a systematic review of peer-reviewed literature, regulatory filings, clinical trial registries, and government policy documents to establish a comprehensive baseline of therapeutic mechanisms, approval landscapes, and guideline recommendations. Proprietary databases and public registries provided data points on product approvals, pricing benchmarks, and tariff schedules. Data triangulation was employed to cross-validate findings, reconcile disparate sources, and preserve the integrity of insights.
Quality assurance processes included iterative reviews by subject-matter experts, consistency checks against established benchmarks, and methodological audits to identify and rectify potential biases. This mixed-methods framework ensures that conclusions drawn and recommendations proposed are grounded in empirical evidence, reflective of real-world contexts, and capable of guiding strategic action in the opioid induced constipation domain.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Opioid Induced Constipation market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Opioid Induced Constipation Market, by Product Type
- Opioid Induced Constipation Market, by Route of Administration
- Opioid Induced Constipation Market, by Distribution Channel
- Opioid Induced Constipation Market, by Region
- Opioid Induced Constipation Market, by Group
- Opioid Induced Constipation Market, by Country
- United States Opioid Induced Constipation Market
- China Opioid Induced Constipation Market
- Competitive Landscape
- List of Figures [Total: 15]
- List of Tables [Total: 954 ]
Summarizing Key Findings and Strategic Considerations to Guide Stakeholders in Navigating the Complexities of Opioid Induced Constipation Management
The management of opioid induced constipation has evolved into a critical dimension of comprehensive pain therapy, driven by patient-centric imperatives, clinical innovation, and shifting trade landscapes. This analysis has highlighted transformative shifts from traditional laxative reliance toward targeted receptor antagonists, underscored by evolving guidelines that favor early, mechanism-based interventions. At the same time, the advent of United States tariffs in 2025 has recalibrated supply chain strategies, compelling manufacturers to reassess sourcing, inventory, and pricing approaches.
Segmentation insights reveal the nuanced interplay between product types and delivery routes, demonstrating the ongoing ascendancy of combination therapies and peripherally acting agents in oral solid and liquid formats, as well as the sustained relevance of rectal administrations in specialized settings. Regional perspectives further emphasize the diversity of market drivers, with the Americas leading in rapid adoption, Europe, Middle East & Africa navigating heterogeneous reimbursement environments, and Asia-Pacific emerging as a dynamic growth frontier.
As competitive dynamics intensify, leading companies continue to differentiate through innovative pipelines, strategic partnerships, and patient support initiatives. The actionable recommendations outlined herein equip stakeholders with a roadmap to optimize portfolios, fortify supply chains, and cultivate favorable payer relationships. By harnessing rigorous research and targeted strategies, organizations can effectively address unmet needs, enhance patient outcomes, and achieve sustainable growth in the opioid induced constipation space.
Driving Informed Decisions: Engage with Ketan Rohom to Access the Definitive Market Research Report on Opioid Induced Constipation and Accelerate Strategic Growth
To secure the comprehensive market research report and harness in-depth insights on evolving treatment modalities, emerging regulatory factors, and competitive dynamics in opioid induced constipation, reach out to Ketan Rohom. As Associate Director, Sales & Marketing, he offers personalized guidance on how the analysis aligns with your strategic objectives and can provide tailored data extracts to inform high-impact decisions. Engage directly with Ketan to explore flexible licensing options, discuss bespoke research deliverables, and accelerate the implementation of evidence-based strategies. Whether you require granular segmentation deep dives, regional adoption benchmarks, or scenario planning for tariff scenarios, Ketan will connect you with the full suite of resources needed to drive market leadership. Initiate a conversation today to transform complex data into actionable intelligence and position your organization at the forefront of opioid induced constipation management innovations

- How big is the Opioid Induced Constipation Market?
- What is the Opioid Induced Constipation Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




