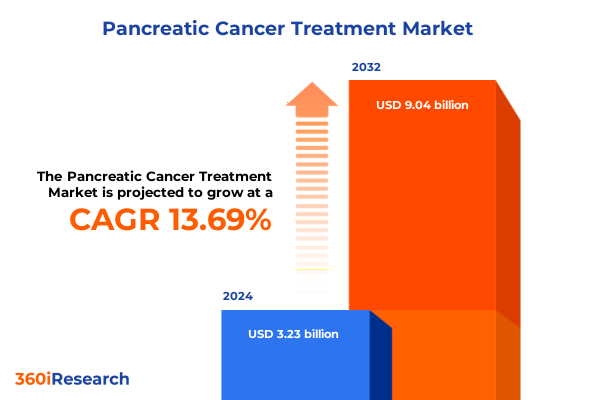
The Pancreatic Cancer Treatment Market size was estimated at USD 3.69 billion in 2025 and expected to reach USD 4.21 billion in 2026, at a CAGR of 13.65% to reach USD 9.04 billion by 2032.
Comprehensive Introduction to the Current Pancreatic Cancer Treatment Ecosystem, Highlighting Unmet Needs and the Drive Toward Precision Oncology Solutions
Pancreatic cancer remains one of the most formidable challenges in oncology, characterized by its aggressive biology, late-stage diagnosis, and historically limited therapeutic options. Despite incremental advances in surgery, chemotherapy, and radiation, the five-year survival rate has shown only marginal improvement over the past decades. This introduction lays the foundation for understanding both the clinical complexity and the urgent unmet needs that continue to drive innovation in treatment modalities.
In response to these challenges, researchers and clinicians are increasingly turning toward precision oncology solutions that leverage molecular profiling, biomarker-driven strategies, and novel immunotherapeutic approaches. By integrating insights from genomics, proteomics, and patient-derived data, the field is moving beyond the one-size-fits-all model toward more tailored interventions. The convergence of these technological breakthroughs with evolving regulatory frameworks signifies a transformative phase in pancreatic cancer care, setting the stage for the in-depth analysis that follows.
Exploring Paradigm Shifts in Pancreatic Cancer Management Driven by Cutting-Edge Therapies, Biomarker Discoveries, and Evolving Patient-Centric Care Models
In recent years, the pancreatic cancer treatment landscape has undergone profound paradigm shifts that extend far beyond incremental variations in standard protocols. Cutting-edge immunotherapies such as chimeric antigen receptor T-cell therapy and next-generation checkpoint inhibitors have begun to challenge the long-standing reliance on cytotoxic chemotherapy. Concurrently, the refinement of vaccine therapy platforms is opening new frontiers in antigen-specific immune activation. These advances are complemented by breakthroughs in targeted therapy, which exploit synthetic lethality and exploit vulnerabilities in DNA damage repair pathways to deliver more precise anticancer activity.
Moreover, the integration of digital health tools and real-world evidence frameworks has accelerated the pace of clinical research and regulatory decision-making. Machine learning algorithms are now being leveraged for early detection, risk stratification, and treatment response prediction. In parallel, patient-centric care models have emerged, emphasizing multidisciplinary collaboration, supportive care optimization, and shared decision-making. By synthesizing these diverse innovations, the field is positioned for sustained transformation in both therapeutic outcomes and overall patient experience.
Assessing the Comprehensive Effects of Newly Implemented 2025 United States Tariffs on the Pancreatic Cancer Treatment Supply Chain and Cost Dynamics
The introduction of new tariffs on imported active pharmaceutical ingredients and specialized medical equipment in 2025 has significantly reshaped the cost structure and supply chain dynamics in pancreatic cancer treatment. Many manufacturers have encountered increased input costs, prompting a reevaluation of procurement strategies and driving deeper collaboration with domestic suppliers. These changes have led to a nuanced rebalancing of production geographies, with some biopharmaceutical companies accelerating investments in onshore API capacity to mitigate future disruptions.
Over time, these cumulative adjustments in sourcing, logistics, and pricing have contributed to shifts in how therapies are distributed and reimbursed. Payers and providers are exploring novel contracting mechanisms, including value-based agreements and risk-sharing models, to manage affordability pressures. At the same time, regulatory agencies and industry associations are engaging in dialogue to streamline import licensing processes and ensure continuity of critical supply chains. As a result, the 2025 tariff environment has catalyzed both cost containment initiatives and strategic realignments across the entire pancreatic cancer treatment ecosystem.
Uncovering In-Depth Segmentation Insights Across Treatment Modalities, Therapy Lines, End Users, and Distribution Pathways Shaping Pancreatic Cancer Care
A nuanced view of the pancreatic cancer treatment arena emerges when examined through multiple segmentation lenses. Based on treatment type, chemotherapy remains foundational, with Capecitabine, Folfirinox, and Gemcitabine-based regimens still widely administered, while novel immunotherapies such as CAR T therapy, checkpoint inhibitors, and vaccine platforms gain traction as complementary or alternative strategies. Palliative care modalities encompassing nutritional support, pain management, and psychological support underscore the importance of quality-of-life measures alongside curative intent. Radiation approaches, from brachytherapy to external beam and stereotactic body radiation therapy, offer dose-precise alternatives when surgical resection is not feasible. Meanwhile, advances in surgical techniques, including distal pancreatectomy, total pancreatectomy, and the Whipple procedure, continue to refine operative outcomes. Finally, targeted therapies leveraging EGFR inhibitors and PARP inhibitors illustrate the shift toward mechanism-driven intervention.
Viewing the market through the therapy line dimension highlights distinct adoption patterns across first-line, second-line, third-line, and fourth-and-above treatments, where safety profiles and incremental efficacy drives determine sequencing decisions. Likewise, end user segmentation distinguishes the roles of ambulatory surgical centers, comprehensive cancer centers, and hospital-based oncology units in delivering different therapeutic modalities. Finally, distribution channels spanning hospital pharmacies, online pharmacies, and retail pharmacies reveal how emerging e-commerce trends and regulatory requirements influence patient access and provider procurement practices.
This comprehensive research report categorizes the Pancreatic Cancer Treatment market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Treatment Type
- Therapy Line
- End User
- Distribution Channel
Analyzing Regional Variations and Growth Drivers in Pancreatic Cancer Treatment across the Americas, EMEA Territories, and the Expanding Asia-Pacific Market
Regional dynamics play a pivotal role in shaping access, reimbursement, and innovation within pancreatic cancer treatment. In the Americas, robust clinical research infrastructure and well-established reimbursement frameworks have supported rapid adoption of novel therapies. The United States leads in venture capital investments for early-stage oncology programs, while Canada and select Latin American markets are developing patient assistance initiatives to bridge affordability gaps. This environment has fostered a competitive landscape where collaboration between academic centers, contract research organizations, and biopharma sponsors accelerates clinical trial enrollment and market entry.
In the Europe, Middle East & Africa region, regulatory harmonization initiatives across European Union member states and targeted access programs in the Middle East have streamlined approval pathways, though variability in national healthcare models continues to affect uptake rates. Meanwhile, sub-Saharan African markets face infrastructure constraints that underscore the need for public-private partnerships and global access programs. Transitioning to the Asia-Pacific landscape, rapid economic growth, expanding healthcare budgets, and government incentives for domestic manufacturing are driving market expansion. Japan and Australia represent mature markets with advanced regulatory ecosystems, while China and India demonstrate exponential growth potential fueled by significant patient populations and emerging biotech hubs. These regional nuances emphasize the importance of tailored strategies to optimize clinical and commercial outcomes.
This comprehensive research report examines key regions that drive the evolution of the Pancreatic Cancer Treatment market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Evaluating Strategic Dynamics and Collaborative Innovations among Leading Biotech and Pharmaceutical Companies Driving Advances in Pancreatic Cancer Treatment
The competitive landscape in pancreatic cancer treatment is defined by a blend of established pharmaceutical giants and agile biotechnology firms. Leading industry players are investing heavily in next-generation checkpoint inhibitors, PARP inhibitors, and CAR T therapies, often through strategic alliances and licensing deals. These collaborations leverage complementary R&D capabilities to accelerate clinical development and broaden the therapeutic pipeline. In parallel, several multinational companies are expanding their presence in precision oncology by acquiring early-stage assets and forging partnerships with diagnostic specialists to integrate companion testing solutions.
At the same time, midsize biotech organizations are distinguishing themselves through targeted vaccine offerings and innovative combination regimens. Their nimble structures enable more rapid pivoting in response to emerging clinical data and shifting regulatory priorities. Contract development and manufacturing organizations have also assumed a critical role, supporting both scale-up and specialized manufacturing of cell and gene therapies. The interplay between these various stakeholders underscores a dynamic ecosystem in which strategic partnerships and disciplined asset management are paramount to securing a leadership position.
This comprehensive research report delivers an in-depth overview of the principal market players in the Pancreatic Cancer Treatment market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- AbbVie Inc.
- Amgen Inc.
- Astellas Pharma Inc.
- AstraZeneca PLC
- Bayer AG
- Bristol-Myers Squibb Company
- Eli Lilly and Company
- Exelixis, Inc.
- F. Hoffmann-La Roche Ltd.
- Genentech, Inc.
- GlaxoSmithKline plc
- Halozyme Therapeutics, Inc.
- Ipsen S.A.
- Johnson & Johnson
- Lisata Therapeutics, Inc.
- Merck & Co., Inc.
- Mirati Therapeutics, Inc.
- Novartis AG
- Novocure GmbH
- Pfizer Inc.
- PharmaCyte Biotech, Inc.
- Sanofi S.A.
- Takeda Pharmaceutical Company Limited
Empowering Industry Leaders with Actionable Strategies to Enhance Innovation, Access, and Patient Outcomes in the Pancreatic Cancer Treatment Arena
To capitalize on emerging opportunities, industry leaders should prioritize investment in precision medicine initiatives, expanding capabilities in genomic profiling and biomarker validation. Aligning internal innovation efforts with real-world evidence generation will enhance value propositions and support differentiated pricing strategies. Additionally, reinforcing supply chain resilience through diversified sourcing agreements and onshore manufacturing partnerships can shield organizations from future trade disruptions and maintain continuity of therapeutic availability.
Furthermore, establishing collaborative agreements with academic institutions, patient advocacy groups, and technology providers will foster integrated care pathways and accelerate talent development. Embracing digital health solutions, such as remote monitoring and AI-driven decision support, can improve patient adherence and clinical outcomes. By adopting these actionable strategies, industry leaders can strengthen competitive positioning, drive sustainable growth, and ultimately contribute to more favorable prognoses for individuals affected by pancreatic cancer.
Detailing the Comprehensive Research Methodology Incorporating Quantitative Analyses, Qualitative Insights, and Data Validation Protocols Underpinning the Study
This research report is grounded in a rigorous methodology that integrates both quantitative and qualitative analyses. Secondary research encompassed a comprehensive review of peer-reviewed journals, regulatory filings, clinical trial registries, and patent databases. Primary research interviews were conducted with oncologists, pharmacoeconomists, supply chain experts, and senior executives from leading pharmaceutical and biotechnology companies to capture firsthand insights on market dynamics and emerging trends.
Data validation protocols included cross-referencing findings with multiple independent sources and triangulating estimates through expert consultations. Advanced analytical techniques were applied to assess segment interrelationships and identify growth drivers. Throughout the study, strict quality control measures ensured consistency and accuracy, enabling stakeholders to confidently base strategic decisions on the evidence presented.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Pancreatic Cancer Treatment market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Pancreatic Cancer Treatment Market, by Treatment Type
- Pancreatic Cancer Treatment Market, by Therapy Line
- Pancreatic Cancer Treatment Market, by End User
- Pancreatic Cancer Treatment Market, by Distribution Channel
- Pancreatic Cancer Treatment Market, by Region
- Pancreatic Cancer Treatment Market, by Group
- Pancreatic Cancer Treatment Market, by Country
- United States Pancreatic Cancer Treatment Market
- China Pancreatic Cancer Treatment Market
- Competitive Landscape
- List of Figures [Total: 16]
- List of Tables [Total: 1749 ]
Synthesizing Key Findings and Strategic Imperatives to Illuminate Pathways for Sustained Progress in Pancreatic Cancer Treatment and Patient Care
As the pancreatic cancer treatment environment continues to evolve, key trends such as the rise of immunotherapies, the integration of targeted therapy, and the adoption of personalized medicine stand out as primary catalysts for change. Supply chain realignments driven by new trade policies underscore the importance of adaptability and strategic forecasting in maintaining uninterrupted therapy access. Furthermore, the multifaceted segmentation analysis highlights how distinct treatment modalities, therapy lines, user settings, and distribution channels each present unique challenges and opportunities.
Collectively, these insights illuminate a path forward that combines scientific innovation with operational excellence. By embracing patient-centered approaches, leveraging partnerships, and embedding flexibility into supply chain and market access strategies, stakeholders are well-positioned to drive meaningful improvements in treatment efficacy and quality of life. The strategic imperatives identified herein serve as guideposts for achieving sustained progress in pancreatic cancer care.
Engage with Ketan Rohom to Secure Exclusive Access to Comprehensive Pancreatic Cancer Treatment Market Intelligence and Drive Informed Decision-Making
To gain unrivaled visibility into the evolving pancreatic cancer treatment landscape and to equip your organization with strategic intelligence that drives competitive advantage, engage directly with Ketan Rohom, Associate Director, Sales & Marketing. His deep expertise in translating complex research insights into actionable business strategies will help you navigate regulatory shifts, optimize research and development priorities, and establish differentiated market positioning. Reach out today to secure bespoke analysis, practical guidance, and executive briefings that will inform critical decisions and accelerate your efforts to improve patient outcomes and organizational growth.

- How big is the Pancreatic Cancer Treatment Market?
- What is the Pancreatic Cancer Treatment Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




