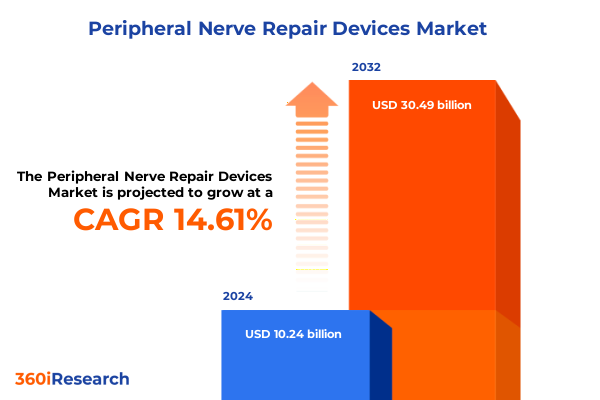
The Peripheral Nerve Repair Devices Market size was estimated at USD 11.71 billion in 2025 and expected to reach USD 13.39 billion in 2026, at a CAGR of 14.64% to reach USD 30.49 billion by 2032.
Unlocking the Critical Imperative of Peripheral Nerve Repair Devices Amidst Rising Clinical Challenges and Opportunities in Regenerative Medicine
Peripheral nerve injuries present a profound clinical challenge, often resulting in significant functional deficits and long-term disability despite complex surgical interventions. The inherent limitations of autografts, such as donor site morbidity and inconsistent regenerative outcomes, expose an urgent need for advanced repair modalities. Conventional strategies continue to underperform when addressing defects beyond short gaps, amplifying the imperative for innovative device-driven solutions that can reliably support axonal regeneration and functional recovery across diverse anatomical sites.
Advancements in biomaterials and tissue engineering have catalyzed a paradigm shift in peripheral nerve repair, introducing functionalized hydrogels, cell-derived extracellular matrices, and conductive scaffolds that actively modulate the regenerative microenvironment. These next-generation materials-engineered to mimic native extracellular cues, deliver growth factors, or facilitate electrical stimulation-demonstrate significant promise in preclinical models for accelerating Schwann cell migration, axonal elongation, and synaptic reconnection.
Alongside biomaterials innovation, regulatory bodies have begun approving a growing portfolio of nerve guidance conduits and allograft products, reflecting a transition from purely mechanical repair constructs toward bioactive platforms that integrate cellular and molecular therapies. This convergence of materials science and clinical translation underscores a maturing ecosystem poised to redefine standards of care for patients with peripheral nerve damage.
Revolutionary Technological Advancements Are Redefining the Peripheral Nerve Repair Landscape Through Biomaterials, Bioelectronics and Regenerative Strategies
The integration of three-dimensional collagen-based nerve guide matrices represents a breakthrough in scaffold design, offering an optimized microarchitecture for Schwann cell migration and directed axonal growth. A pioneering example is the NeuraGen 3D Nerve Guide Matrix, which couples bovine Type I collagen conduits with an internal architecture that recreates endoneurial pathways, demonstrating enhanced functional outcomes over traditional hollow tubes.
Simultaneously, conductive biomaterials have emerged as a key transformative shift, harnessing electrical stimulation to augment neuroregenerative processes. Recent reviews indicate that conductive polymers incorporated into nerve conduits facilitate bioelectric signal transmission and improve neurite extension under controlled stimulation, thereby bridging the gap between bioengineering innovation and electrophysiological recovery.
Complementing these advances, functionalized hydrogels and cell-derived matrices have shown the capacity to actively modulate inflammation, deliver trophic factors, and recruit endogenous cell populations. Such systems provide a versatile platform for integrating multiple regenerative cues-combining controlled degradation kinetics with localized bioactive molecule release-to create a dynamic repair niche that accelerates axonal regeneration and functional reinnervation in preclinical peripheral nerve injury models.
Assessing the Far-Reaching Consequences of Newly Imposed 2025 U.S. Tariffs on the Peripheral Nerve Repair Device Supply Chain and Manufacturing Ecosystem
In May 2025, the U.S. Trade Representative reinstated Section 301 tariffs on Class I and II medical devices imported primarily from China, encompassing a broad range of peripheral nerve repair instruments and consumables. This policy shift introduces significant cost pressures for device manufacturers, compelling rapid supplier diversification and prompting strategic discussions on resilient, regionally distributed production models within the medtech industry.
Medical device trade associations, including AdvaMed, have lobbied aggressively for carve-outs to safeguard device affordability and maintain patient access. Hospital groups echo these concerns, warning that elevated import duties will disproportionately strain fixed reimbursement contracts and hospital budgets, ultimately translating into higher out-of-pocket costs for patients and reduced availability of specialized nerve repair technologies.
Leading medtech companies have quantified the financial impact, with Zimmer Biomet projecting a $60–80 million reduction in 2025 profits and Johnson & Johnson estimating a $400 million headwind for its MedTech division. These projections underscore a broader industry imperative to optimize supply chains through nearshoring, tariff engineering, and renegotiated supplier agreements to mitigate margin erosion and ensure continuity of device innovation and availability.
In-Depth Analysis Reveals How Product Types, Cutting-Edge Technologies and Clinical Applications Shape the Segmented Peripheral Nerve Repair Devices Market
Market segmentation by product type reveals a nuanced landscape in which nerve conduits, nerve grafts, nerve wraps, and neurotrophic factor formulations each address distinct clinical needs. Among these, nerve grafts-encompassing allografts, autografts, and xenografts-remain a cornerstone for managing moderate to large nerve gaps, benefitting from established surgical protocols and favorable regenerative outcomes in well-defined anatomical contexts.
Within technology segmentation, biodegradable polymers such as polycaprolactone, polyglycolide, and polylactic acid dominate research activity and clinical translation efforts. Collagen-based conduits leveraging Type I and III collagen continue to expand in clinical use, while synthetic polymers like polyethylene terephthalate and polyurethane offer mechanical tuning and tailored degradation profiles. Recent studies highlight the successful integration of these materials into advanced conduit designs that optimize porosity, mechanical strength, and biomolecule incorporation for improved nerve regeneration outcomes.
Repair site segmentation underscores the heterogeneity of peripheral nerve injuries, with cranial nerve reconstructions demanding ultra-fine precision and microsuturing, while upper and lower extremity nerve repairs benefit from robust conduit scaffolds. Procedure type segmentation shows endoscopic and microsurgical approaches as the current standards, with robotic-assisted microsurgery gaining momentum due to its precision and minimized tremor. Concurrently, application-based segmentation differentiates chronic neuropathies, acute traumatic injuries, and reconstructive surgeries, each driving unique device requirements. Finally, end-user segmentation confirms hospitals, ambulatory surgical centers, and research institutes as critical adoption channels that shape device design, sterilization standards, and service support models.
This comprehensive research report categorizes the Peripheral Nerve Repair Devices market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Product Type
- Technology
- Repair Site
- Procedure Type
- Application
- End User
Regional Dynamics Unveiled as Americas, EMEA and Asia-Pacific Markets Navigate Regulatory, Economic and Technological Drivers in Peripheral Nerve Repair Devices
The Americas region, led by the United States, maintains its dominance in peripheral nerve repair devices through robust healthcare spending, established reimbursement frameworks, and a strong network of research hospitals and ambulatory surgical centers. High adoption rates of advanced conduits and graft materials are supported by favorable regulatory pathways and domestic manufacturing capabilities, which buffer the impact of international tariff fluctuations.
In Europe, Middle East & Africa, evolving regulatory frameworks-most notably the European Medical Device Regulation-are reshaping device classification, clinical evidence requirements, and post-market surveillance. European exporters face heightened cost pressures from U.S. tariffs, estimated at an additional €3.3 billion in annual export duties, driving strategic relocation considerations and a pivot toward emerging markets in Latin America and Asia to sustain growth and preserve innovation investment.
Asia-Pacific emerges as the fastest-growing region, propelled by rising incidence of nerve injuries, expanding surgical infrastructure, and government-led initiatives to enhance domestic manufacture of advanced medical technologies. Countries such as China, India, and Japan are investing heavily in neuroregenerative research consortia and localizing production of both conduits and bioactive factors, thereby establishing a critical axis for global supply chain diversification and clinical trial activity in peripheral nerve repair.
This comprehensive research report examines key regions that drive the evolution of the Peripheral Nerve Repair Devices market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Strategic Profiles of Leading Peripheral Nerve Repair Device Manufacturers Highlight Innovations, Collaborations and Competitive Differentiators Shaping the Industry
Major stakeholders in the peripheral nerve repair devices arena encompass both global medtech giants and specialized innovators. Abbott Laboratories, Medtronic, and Stryker leverage extensive distribution networks and regulatory expertise to commercialize a spectrum of conduits and graft products. Companies like Axogen and Integra LifeSciences focus on biologic scaffolds and resorbable collagen matrices, while Polyganics and Toyobo advance synthetic polymer conduits designed for customized degradation and mechanical performance.
Collagen Matrix Inc. distinguishes itself through a robust portfolio of Type I collagen wraps and nerve protectors, demonstrating clinical efficacy in adhesion prevention and enhanced nerve gliding. NeuroPace and SPR Therapeutics, although primarily known for neuromodulation, are exploring integrated repair solutions that combine electrical stimulation with traditional nerve repair constructs. Partnerships between tech startups and established manufacturers, exemplified by Stryker’s collaboration with MMI on the Symani® Surgical System for microsurgical applications, illustrate a convergence of device innovation and surgical robotics to address precision repair needs.
In addition, mid-size players and regional specialists such as Regenity, Synovis Micro Companies Alliance, and Collagen Matrix Inc. drive competitive differentiation through niche product lines, targeted R&D agendas, and flexible commercial models. Their ability to rapidly iterate on conduit materials and integrate biofunctional additives underscores a dynamic competitive landscape in which strategic acquisitions and co-development agreements are key catalysts for portfolio expansion and market penetration.
This comprehensive research report delivers an in-depth overview of the principal market players in the Peripheral Nerve Repair Devices market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Abbott Laboratories
- Axogen, Inc.
- B. Braun SE
- Baxter International Inc.
- BioCircuit Technologies, Inc.
- Boston Scientific Corporation
- Checkpoint Surgical, Inc.
- Integra LifeSciences Holdings Corporation
- LivaNova PLC
- Medtronic PLC
- MicroTransponder Inc.
- NervGen Pharma Corp.
- Neuraptive Therapeutics, Inc.
- NeuroPace, Inc.
- Newrotex
- Orthocell Ltd.
- Regenity Biosciences
- ReNerve Pty Ltd
- Saluda Medical Pty Ltd.
- Stryker Corporation
- TOYOBO CO., LTD.
Actionable Recommendations Arise for Industry Leaders to Drive Innovation, Optimize Supply Chains and Amplify Market Growth in Peripheral Nerve Repair Devices
To navigate complex tariff environments and mitigate supply chain vulnerabilities, industry leaders should proactively diversify manufacturing footprints across low-tariff regions, leveraging nearshore sites in Mexico and Canada under USMCA frameworks, while cultivating strategic partnerships for regional production in Asia-Pacific hubs.
Investment in advanced biodegradable polymer R&D-particularly focusing on polylactic-co-glycolic acid composites and shape-memory polymers-will differentiate product pipelines and address the clinical demand for customized degradation profiles tailored to specific nerve gap lengths and healing trajectories.
Collaboration with academic and clinical research centers to establish post-market clinical registries and real-world evidence platforms will enhance reimbursement positioning and facilitate accelerated regulatory approvals, while demonstrating longitudinal safety and efficacy in diverse patient populations. Simultaneously, integrating data from neurostimulation and bioelectronic interfaces can enrich product portfolios and unlock novel therapeutic pathways in nerve repair and neuromodulation convergence.
Comprehensive Research Methodology Combining Rigorous Secondary Data, Expert Interviews and Robust Validation Techniques Ensures High-Quality Insights
The research underpinning this report employs a multi-stage approach, beginning with exhaustive secondary research across peer-reviewed journals, industry publications, regulatory filings, and company disclosures. Key databases accessed include MEDLINE, PubMed, and clinical trials registries to map technological trends and clinical evidence profiles.
Primary research involved in-depth interviews with medtech executives, surgical specialists, procurement officers, and regulatory experts to validate secondary data, uncover strategic imperatives, and gauge adoption drivers. This qualitative input was triangulated with quantitative insights to ensure comprehensiveness and accuracy.
Data validation incorporated cross-comparison of company product pipelines, patent filings, and published clinical outcomes, augmented by scenario analyses to assess the impact of geopolitical shifts, tariff changes, and emerging reimbursement policies. Rigorous quality control checks guarantee that conclusions reflect the most up-to-date and reliable industry intelligence.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Peripheral Nerve Repair Devices market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Peripheral Nerve Repair Devices Market, by Product Type
- Peripheral Nerve Repair Devices Market, by Technology
- Peripheral Nerve Repair Devices Market, by Repair Site
- Peripheral Nerve Repair Devices Market, by Procedure Type
- Peripheral Nerve Repair Devices Market, by Application
- Peripheral Nerve Repair Devices Market, by End User
- Peripheral Nerve Repair Devices Market, by Region
- Peripheral Nerve Repair Devices Market, by Group
- Peripheral Nerve Repair Devices Market, by Country
- United States Peripheral Nerve Repair Devices Market
- China Peripheral Nerve Repair Devices Market
- Competitive Landscape
- List of Figures [Total: 18]
- List of Tables [Total: 1749 ]
Concluding Perspectives Emphasize the Imperative of Continued Innovation, Adaptive Strategies and Collaborative Efforts in Peripheral Nerve Repair Advancement
The peripheral nerve repair devices landscape is characterized by a convergence of advanced biomaterials, regenerative therapies, and surgical innovations that collectively promise to transform patient outcomes. From collagen-based conduits to conductive scaffolds and functionalized hydrogels, the pace of technological evolution underscores the sector’s potential to address longstanding clinical gaps.
Geopolitical and economic forces, notably the reinstated U.S. tariffs, accentuate the need for agile supply chain strategies, regional manufacturing diversification, and proactive policy engagement to safeguard device affordability and availability. Market segmentation insights reinforce the importance of targeting specific clinical applications, technological platforms, and end-user channels to optimize commercialization strategies.
As leading companies refine product portfolios through strategic collaborations, polymer innovations, and integrated bioelectronic approaches, the market stands at a pivotal juncture. Embracing actionable recommendations and leveraging high-quality market intelligence will be critical for stakeholders aiming to navigate complexity and realize the full potential of peripheral nerve repair device solutions.
Engage with Ketan Rohom to Secure Customized Peripheral Nerve Repair Device Market Intelligence and Unlock Strategic Insights for Informed Decision-Making
Partnering with our expert Associate Director of Sales & Marketing, Ketan Rohom, unlocks a tailored path to the critical market intelligence needed to outmaneuver competition in the increasingly dynamic realm of peripheral nerve repair devices. His deep understanding of surgical device trends, regulatory nuances, and regional market dynamics ensures that you receive customized insights aligned with your strategic objectives. By engaging directly, you gain access to timely updates on technological innovations, tariff impacts, and segmentation shifts that influence procurement, product development, and investment decisions. Seize the opportunity to enhance your competitive advantage with a comprehensive, actionable report that informs product portfolio optimization, market entry strategies, and partnership evaluations. Reach out now to secure your copy and embark on a data-driven journey toward transformative growth in the peripheral nerve repair devices market

- How big is the Peripheral Nerve Repair Devices Market?
- What is the Peripheral Nerve Repair Devices Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




