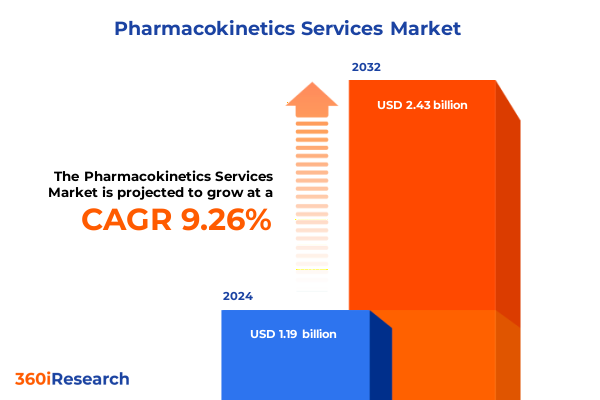
The Pharmacokinetics Services Market size was estimated at USD 1.30 billion in 2025 and expected to reach USD 1.41 billion in 2026, at a CAGR of 9.34% to reach USD 2.43 billion by 2032.
Unveiling the Strategic Imperative of Pharmacokinetics Services for Optimizing Drug Development Timelines and Enhancing Therapeutic Outcomes
Pharmacokinetics represents a fundamental cornerstone of drug discovery and development, elucidating the intricate journey a therapeutic compound undergoes from administration to elimination. This discipline examines the four key processes of absorption, distribution, metabolism, and excretion (ADME), providing indispensable insights into how a molecule behaves within living systems and ensuring that dosing regimens are both safe and efficacious. Understanding these ADME parameters early and throughout development reduces clinical attrition by identifying variability factors that could compromise safety or therapeutic outcomes, ultimately streamlining the path from bench to bedside.
Equally critical is the role of pharmacokinetic studies in informing regulatory submissions and guiding subsequent clinical trial design. By characterizing drug–drug and drug–food interactions, and assessing the impact of organ impairment on drug exposure, these investigations furnish the data necessary to predict human responses, optimize dosing strategies, and meet stringent approval requirements. The ability to gather robust PK data across diverse populations and stages of development underpins confidence in downstream clinical performance and real-world application, thereby increasing the likelihood of success for novel therapies.
Charting the Major Technological and Process Innovations Revolutionizing Pharmacokinetics Services and Drug Development Efficiencies
The pharmacokinetics services landscape is undergoing profound transformation driven by the rapid integration of digital technologies and artificial intelligence. AI-driven analytics now enable the processing of vast datasets to predict drug behavior and patient-specific responses, significantly reducing traditional trial timelines. Cloud-based platforms facilitate real-time collaboration across research teams, enhancing transparency and accelerating decision-making. These advancements are complemented by the adoption of machine learning algorithms to refine ADME predictions, optimizing lead selection and minimizing costly late-stage failures.
Simultaneously, the laboratory environment is evolving through extensive automation and Internet of Medical Things (IoMT) integration. Automated sample handling and high-throughput screening systems drive reproducibility and efficiency in preclinical ADME assays, while remote monitoring tools ensure compliance and data integrity. The next wave of innovation is poised to leverage connected devices for continuous in vivo sampling, offering unprecedented granularity in exposure profiling.
Moreover, personalized medicine is gaining traction as pharmacokinetic protocols increasingly incorporate biomarker-driven stratification. AI-powered biomarker discovery accelerates identification of metabolic phenotypes, enabling customized dosing regimens that enhance safety and efficacy. Real-time monitoring devices now provide dynamic feedback on drug concentrations in patients, fostering adaptive dosing strategies that optimize therapeutic windows. This convergence of digital health, advanced modeling, and precision dosing heralds a new era in pharmacokinetics services.
Analyzing the Broad Financial and Operational Repercussions of 2025 U.S. Tariff Policies on Pharmacokinetics Service Supply Chains and Protocol Costs
In early 2025, the United States implemented broad tariff measures that now impose a 10% global duty on all imported goods, with pharmaceutical products subject to additional levies. These policies include a 25% tariff on finished pharmaceutical imports and sharply higher duties on APIs sourced from certain countries. While intended to bolster domestic manufacturing, these measures have led to increased raw material costs and procedural delays for pharmacokinetics service providers that rely on global supply chains. Companies face heightened pressure to reassess vendor agreements and adapt sourcing strategies to mitigate escalating expenses.
The ripple effects of these tariffs extend to generic drug developers and testing laboratories, which often operate on thin margins and depend heavily on imported components. With API costs rising by as much as 10% for Chinese-sourced ingredients, many organizations are exploring alternative suppliers or reshoring certain operations. However, building domestic capacity requires significant investment and time, underscoring the urgency for strategic planning to maintain service continuity and affordability for end users.
Revealing Distinct Market Segments to Illustrate Differential Demand Patterns Across Pharmacokinetics Service Modalities and Client Profiles
Understanding service demand through the lens of molecule type reveals clear distinctions in pharmacokinetics requirements. Large molecule services, such as those supporting biologics, necessitate immunoassay development and specialized mass spectrometry to capture complex macromolecular behavior, while small molecule workflows leverage high-throughput chromatographic assays for rapid quantification. These divergent technical needs shape resource allocation, instrumentation investments, and expert skill sets within service providers.
Service type segmentation further clarifies market dynamics, as in vitro platforms are prized for mechanism-focused assessments and permeability predictions, offering cost-effective, high-throughput screening during early discovery stages. In contrast, in vivo studies provide systemic exposure profiles essential for translational relevance, allowing for the establishment of dose–exposure relationships in animal models that support human dose projections. The balance between these methodologies influences project timelines, risk profiles, and regulatory pathways.
End-user segmentation drives tailored offerings, with academic and government research institutes pursuing mechanistic insights and methodological innovation; biotechnology companies requiring flexible, scalable workflows to support nimble pipeline development; contract research organizations prioritizing integrated solutions and rapid turnaround for sponsor satisfaction; and large pharmaceutical companies seeking standardized, fully validated packages compliant with global regulatory standards. Each user category imposes distinct performance metrics and service-level expectations on providers.
This comprehensive research report categorizes the Pharmacokinetics Services market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Type
- Service Type
- End-User
Uncovering Regional Dynamics and Growth Drivers Shaping the Pharmacokinetics Services Market Across Americas Europe Middle East & Africa and Asia Pacific
In North America, the United States dominates pharmacokinetics services, driven by a robust regulatory framework and a high concentration of leading pharmaceutical developers partnering with specialized CROs for ADME and PKPD studies. Strategic alliances between drug manufacturers and service providers ensure end-to-end support for early discovery through late-stage clinical evaluation, leveraging comprehensive data to secure regulatory approvals swiftly.
Europe, Middle East & Africa showcases a diverse market shaped by the European Medicines Agency’s harmonized guidelines and the increasing outsourcing of preclinical ADME and human PK studies to CROs across Germany, France, and the UK. Emerging biotech hubs in the Middle East and South Africa are supplementing established markets, capitalizing on regulatory convergence and lower operating costs, while Europe’s extensive CRO infrastructure continues to attract global partnerships.
Asia-Pacific represents the fastest-growing regional segment, characterized by accelerated CRO expansion in China, India, and Japan. Governments in these economies are incentivizing foreign investment and technology transfer, resulting in extensive adoption of big data analytics for PK modeling and rapid scale-up of in vitro and in vivo platforms. Cost efficiencies and access to large patient populations for clinical studies further drive market momentum.
This comprehensive research report examines key regions that drive the evolution of the Pharmacokinetics Services market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Highlighting Leading Global Pharmacokinetics Service Providers and Their Unique Value Propositions in a Competitive Landscape
Global pharmacokinetics service leaders distinguish themselves through breadth of expertise, geographic reach, and integrated offerings. Charles River Laboratories, Eurofins Scientific, and Evotec provide extensive bioanalytical and ADME profiling platforms, underpinned by state-of-the-art mass spectrometry and immunoassay capabilities. Certara differentiates through advanced modeling and simulation software that informs PKPD relationships, while Parexel and Thermo Fisher Scientific leverage their broad service portfolios to offer seamless transitions from preclinical assays to clinical trial support. Each of these organizations maintains rigorous quality systems and global compliance frameworks that reinforce trust among large pharmaceutical sponsors.
Complementing these established players are specialized providers innovating niche solutions. Biologics Consulting Group, Creative Bioarray, and GVK Biosciences cater to complex biologic and cell therapy pipelines with targeted assay development, while Pacific BioLabs and Frontage Laboratories emphasize flexible project management and rapid turnaround. This diverse ecosystem ensures that sponsors can access tailored service combinations aligned with their unique molecule characteristics and development strategies.
This comprehensive research report delivers an in-depth overview of the principal market players in the Pharmacokinetics Services market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Absorption Systems LLC
- Allucent
- Aurigene Pharmaceutical Services Ltd.
- Certara
- Charles River Laboratories, Inc.
- Creative Bioarray
- Eurofins Scientific SE
- Evotec SE
- Frontage Laboratories, Inc.
- ICON PLC
- Laboratory Corporation of America Holdings
- LGC Limited by Cinven
- NUVISAN Pharma Holding GmbH
- Pacific BioLabs
- Parexel International (MA) Corporation
- Pfizer Inc.
- PPD Inc. by Thermo Fisher Scientific Inc.
- Premier Consulting
- Reaction Biology Corporation
- SGS SA
- Svar Life Science AB
- WuXi AppTec Co., Ltd.
- XenoTech by BioIVT
- Xyzagen
Offering Strategic Recommendations to Empower Industry Leaders to Capitalize on Emerging Opportunities and Mitigate Operational Risks
To capitalize on evolving market conditions, organizations should accelerate digital integration by investing in AI-driven analytics and cloud-enabled informatics platforms. Embracing automated workflows and continuous monitoring tools can enhance data quality while reducing sample handling errors and cycle times. Establishing cross-functional teams dedicated to digital transformation will ensure alignment of technological investments with scientific objectives and regulatory requirements.
Simultaneously, industry leaders must develop resilient supply chains by diversifying raw material suppliers and exploring near-shore or on-shore manufacturing partnerships to mitigate tariff-related cost pressures. Collaborative alliances with local authorities and participation in policy dialogues can further influence trade discussions and secure tariff exemptions for critical pharmacokinetic reagents. Integrating these strategies will position organizations to maintain operational continuity and control cost trajectories in an uncertain trade environment.
Articulating a Robust Multi-Tiered Research Methodology Incorporating Primary and Secondary Data Sources and Rigorous Data Triangulation Techniques
Our research framework combined exhaustive secondary research with rigorous primary validation to ensure comprehensive market coverage and data integrity. Secondary sources included peer-reviewed scientific journals, regulatory directives from agencies such as the FDA and EMA, company white papers, industry conference proceedings, and global trade databases. These inputs established foundational insights into market structure, technological trends, and regulatory shifts.
Primary research efforts encompassed in-depth interviews with pharmacokinetics experts, CRO leadership, and regulatory affairs specialists, supplemented by surveys targeting R&D heads and procurement managers. Through iterative data triangulation, we cross-verified quantitative findings with qualitative perspectives, identifying potential discrepancies and refining assumptions. Final validation workshops with key opinion leaders ensured that our conclusions and recommendations accurately reflect current and anticipated market dynamics.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Pharmacokinetics Services market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Pharmacokinetics Services Market, by Type
- Pharmacokinetics Services Market, by Service Type
- Pharmacokinetics Services Market, by End-User
- Pharmacokinetics Services Market, by Region
- Pharmacokinetics Services Market, by Group
- Pharmacokinetics Services Market, by Country
- United States Pharmacokinetics Services Market
- China Pharmacokinetics Services Market
- Competitive Landscape
- List of Figures [Total: 15]
- List of Tables [Total: 636 ]
Synthesizing Key Insights to Emphasize the Strategic Imperatives and Future Outlook for Pharmacokinetics Service Stakeholders
In summary, the pharmacokinetics services sector stands at a transformative juncture, propelled by digital innovations, evolving regulatory landscapes, and shifting trade policies. Providers that strategically integrate AI-powered analytics, automation, and advanced modeling will gain a decisive edge in delivering high-quality ADME and PKPD insights. Resilient supply chain strategies and diversified service portfolios will be critical to sustaining growth amidst tariff uncertainties and changing end-user needs.
Looking ahead, the convergence of personalized medicine approaches with real-time monitoring technologies promises to redefine the parameters of pharmacokinetic evaluation. Organizations that foster collaborative partnerships, invest in emerging market capabilities, and continuously adapt their service models to reflect scientific and policy developments will be best positioned to lead in this dynamic and essential segment of drug development.
Seize the Opportunity Today by Engaging with Associate Director of Sales & Marketing to Secure Your Comprehensive Pharmacokinetics Market Report
We invite industry stakeholders to take decisive action and obtain the full pharmacokinetics services market research report by reaching out directly to Ketan Rohom, Associate Director of Sales & Marketing. This comprehensive analysis offers the detailed insights and strategic guidance necessary to inform investment decisions, enhance service offerings, and drive competitive advantage. By securing your copy, you gain access to meticulously curated data across market dynamics, regulatory impacts, and technology trends, empowering your organization to navigate challenges and capitalize on emerging opportunities with confidence.
To begin the process, simply connect with Ketan Rohom to discuss your unique business needs and discover the customizable options available for report delivery. Whether you’re aiming to refine your R&D strategy, explore new service lines, or mitigate supply chain risks, his expertise will ensure you receive a timely, tailored solution that aligns with your strategic objectives. Seize this opportunity to accelerate your decision-making and reinforce your market leadership-contact Ketan Rohom today to secure the insights that will drive your success.

- How big is the Pharmacokinetics Services Market?
- What is the Pharmacokinetics Services Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




