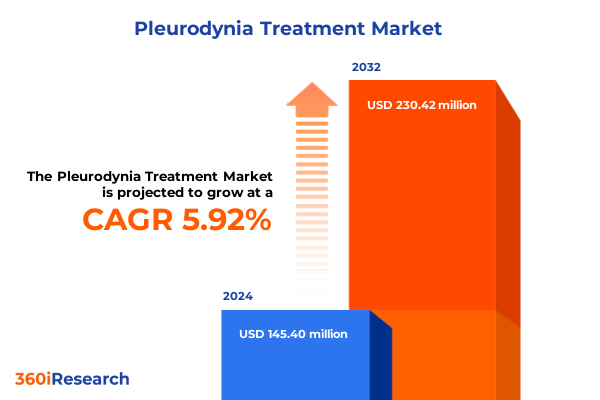
The Pleurodynia Treatment Market size was estimated at USD 153.87 million in 2025 and expected to reach USD 163.52 million in 2026, at a CAGR of 5.93% to reach USD 230.42 million by 2032.
Exploring the Critical Role of Pleurodynia Treatment as an Emerging Priority in Pain Management and Viral Care Worldwide
Pleurodynia, often characterized by sudden and severe chest wall pain, represents an acute infection typically caused by enteroviruses such as coxsackievirus B. The condition manifests with episodic spasms of the intercostal muscles and can create significant discomfort that interferes with everyday activities. Despite its generally self-limiting nature, pleurodynia’s unpredictable pain and the potential for fever and systemic symptoms drive demand for effective therapeutic interventions. Clinicians and patients alike seek options that not only alleviate acute pain but also address inflammatory and viral pathways to reduce symptom duration.
In recent years, advances in pharmacology have led to broader interest in combination therapies that integrate analgesic, antipyretic, and antiviral agents to deliver a more comprehensive approach to care. This evolution reflects a growing appreciation for the multifaceted pathology of pleurodynia and the need for personalized treatment regimens. As healthcare systems emphasize value-based care, payers and providers are evaluating treatments for both efficacy and cost-effectiveness, giving rise to new clinical guidelines that prioritize rapid symptom control alongside supportive management.
With rising awareness of antiviral strategies and the emergence of novel drug formulations, industry stakeholders are at a pivotal moment. Regulatory bodies are issuing updated guidance to streamline approvals for combination products, and specialty pharmacies are expanding their role in managing complex treatment protocols. Amid these developments, a deeper understanding of treatment dynamics is critical for manufacturers, healthcare organizations, and policymakers aiming to optimize patient outcomes and navigate a shifting therapeutic landscape.
Unveiling the Transformative Shifts Steering Pleurodynia Treatment with Breakthrough Therapies and Evolving Patient Care Paradigms
The treatment landscape for pleurodynia is undergoing transformative shifts driven by innovations in drug development and evolving patient expectations. Recent years have seen a surge in the investigation of targeted antiviral agents that aim to shorten the duration of viral replication, thereby mitigating the severity of pleurodynia outbreaks. Concurrently, the analgesics segment has diversified beyond traditional nonsteroidal anti-inflammatory drugs to include novel molecules that modulate inflammatory pathways with improved safety profiles.
Moreover, combination therapies that merge antiviral compounds with analgesic and antipyretic agents are redefining standard care approaches. Such integrative formulations address multiple symptomatic dimensions in a single dosage, reducing pill burden and enhancing adherence. Digital health solutions are likewise making a significant impact; remote monitoring of pain episodes and teleconsultation platforms enable real-time adjustments to therapy, ensuring that treatment regimens remain aligned with patient needs.
Further changes are evident in reimbursement frameworks that increasingly reward outcomes over volume. Payers are negotiating performance-based agreements that hinge on rapid pain relief and reduced hospitalization rates, encouraging manufacturers to demonstrate real-world effectiveness. As telemedicine expands access in both urban and rural settings, treatment protocols are adapting to enable home-based administration of injectable antivirals and extended-release formulations. These converging trends underscore a dynamic market environment where agility and innovation will drive the next generation of pleurodynia care.
Assessing the Far-Reaching Consequences of United States Tariff Changes in 2025 on Pleurodynia Treatment Supply Chains and Pricing Dynamics
In 2025, newly implemented tariff adjustments in the United States have introduced notable complexities for manufacturers and distributors of pleurodynia treatments. Increased duties on imported active pharmaceutical ingredients have heightened production costs for analgesics and antivirals alike, compelling many firms to reevaluate global supply chains. For organizations reliant on specialized excipients and viral vectors sourced abroad, the impact has translated into extended lead times and tighter inventory buffers.
These cost pressures have been compounded by parallel measures targeting imported packaging materials and prefilled syringe components. With domestic capacity for high-quality sterile manufacturing still scaling up, some producers have faced difficult choices between passing on higher costs to healthcare providers or absorbing margins to maintain competitive pricing. Consequently, profit erosion in certain segments has led to consolidation among mid-sized suppliers and spurred partnerships that seek to localize critical production stages.
Looking ahead, stakeholders are engaging with regulatory authorities to secure tariff exemptions for essential raw materials, arguing that pleurodynia treatments constitute a public health necessity. Expanded investments in domestic API manufacturing and increased collaboration with contract development and manufacturing organizations suggest a strategic pivot toward resilience. As these initiatives unfold, companies that proactively optimize their supply networks and leverage tariff mitigation strategies will be best positioned to sustain operational continuity and deliver uninterrupted access to pleurodynia therapies.
Key Segmentation Insights Illuminating Diverse Treatment Classes Dosage Forms Distribution Channels and End User Endpoints in Pleurodynia Care
Insights emerging from treatment class segmentation reveal that analgesics remain foundational to pleurodynia management, with nonsteroidal anti-inflammatory drugs being the most widely used due to their well-understood efficacy and safety profile. However, opioid analgesics continue to play a targeted role for patients experiencing refractory pain, despite regulatory scrutiny and an emphasis on minimizing misuse. Antipyretic therapies are also integral, with ibuprofen and paracetamol serving as frontline options to control fever and associated discomfort. The expansion of antiviral agents adds a new dimension, offering potential to shorten symptom duration and reduce viral shedding when administered early in the disease course. Combination therapies that integrate these modalities provide a cohesive framework for addressing both symptomatic relief and underlying viral activity.
When examining dosage form preferences, injectables are gaining traction, particularly prefilled syringes that offer precise dosing and enhanced convenience for healthcare professionals and patients in outpatient settings. Vials remain a cost-effective option for hospitals and clinics with robust compounding capabilities. Meanwhile, suspensions and solutions cater to pediatric populations and individuals with swallowing difficulties, with formulations engineered for palatability and stability. Tablets and capsules, representing the most traditional delivery mechanism, are diversifying into extended-release profiles that maintain consistent plasma concentrations and reduce dosing frequency.
From a distribution standpoint, hospital pharmacies-both government and private-continue to dominate high-acuity care settings, leveraging established procurement channels to maintain inventory. Online pharmacies are rapidly expanding their share through e-marketplaces and specialized pharmaceutical e-retail platforms, providing home delivery options that align with patient preferences for convenience. Meanwhile, chain and independent retail pharmacies are enhancing their role by offering pharmacist-led counseling and administering injectables under collaborative practice agreements.
End users span ambulatory surgery centers where rapid relief protocols are critical, alongside clinics of both general practice and specialty orientation that manage a broad spectrum of respiratory and thoracic conditions. Home care administration is increasingly supported by caregiver training programs and self-administration kits, enabling patients to receive complex therapies outside institutional settings. Hospitals, both public and private, remain central to managing severe cases, integrating pleurodynia care into broader inpatient treatment pathways.
This comprehensive research report categorizes the Pleurodynia Treatment market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Treatment Class
- Dosage Form
- Distribution Channel
- End User
Uncovering Key Regional Insights Highlighting Distinct Market Drivers and Opportunities across the Americas EMEA and Asia-Pacific Regions
In the Americas region, the United States remains at the forefront of pleurodynia treatment innovation, with strong clinical trial activity and well-developed reimbursement systems that support advanced combination therapies. Canada is following closely, bolstered by public-private partnerships that foster domestic production of antiviral compounds. Brazil and Mexico are emerging markets, with rising investments in injectable formulations and growing adoption of telemedicine services for remote patient monitoring.
Across Europe, the Middle East, and Africa region, regulatory harmonization efforts within the European Union have streamlined approval pathways for new pleurodynia treatments, encouraging multinational manufacturers to prioritize launches in key European markets. In the Middle East, health authorities are enhancing local manufacturing capabilities through joint ventures, while parts of Africa are witnessing pilot programs that introduce mobile health clinics to deliver injectable antivirals in rural areas.
Asia-Pacific markets exhibit diverse growth trajectories. Japan’s stringent regulatory standards are matched by high patient expectations for safety and efficacy, driving demand for premium combination products. China is rapidly expanding its generic manufacturing base while increasing funding for novel antivirals. India, leveraging its strong pharmaceutical industry, is playing a pivotal role in global supply chains, particularly for paracetamol and ibuprofen. Meanwhile, emerging economies in Southeast Asia are adopting regulatory frameworks that facilitate faster market entry for innovative therapies.
Collectively, these regional dynamics underscore the importance of tailoring commercial strategies to reflect local regulatory environments, healthcare infrastructure maturity, and patient access programs. Companies that adapt their portfolios with region-specific formulations and distribution partnerships will be best positioned to capture growth across these varied landscapes.
This comprehensive research report examines key regions that drive the evolution of the Pleurodynia Treatment market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Profiling Leading Companies Driving Innovation in Pleurodynia Treatment from Analgesic Manufacturers to Antiviral and Combination Therapy Specialists
Leading pharmaceutical companies are investing heavily in pleurodynia treatment portfolios to address unmet clinical needs and capture growing demand for integrated care solutions. Global analgesics leaders have launched next-generation NSAID formulations that offer improved gastrointestinal tolerability, while specialty biotech firms are advancing novel antiviral candidates through late-stage clinical trials. Strategic alliances between large-cap manufacturers and nimble research-focused organizations have accelerated the development of combination therapies that marry rapid pain relief with antiviral efficacy.
Mid-sized players are carving out niches by focusing on specialized dosage forms, such as extended-release injectable platforms and pediatric-friendly suspensions. These companies leverage agility to navigate regulatory processes efficiently and partner with contract development and manufacturing organizations to scale production rapidly. Meanwhile, regional pharmaceutical groups in Asia and Latin America are seizing on local manufacturing capabilities to supply cost-competitive generic antipyretic and analgesic products, reinforcing the balance between affordability and quality.
Emerging digital health companies are also influencing the competitive landscape by offering telemedicine platforms that integrate treatment adherence monitoring and real-time symptom tracking. Such platforms enable pharmaceutical companies to gather valuable real-world evidence and refine product offerings accordingly. Additionally, several contract research organizations and consultancy firms are enhancing their service portfolios to include market access advisory, ensuring that new pleurodynia therapies navigate pricing negotiations and reimbursement submissions effectively.
Overall, the competitive environment is characterized by a blend of large-scale innovators, focused specialty firms, and technology-enabled service providers, all collaborating and competing to deliver comprehensive pleurodynia care solutions.
This comprehensive research report delivers an in-depth overview of the principal market players in the Pleurodynia Treatment market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- AbbVie Inc.
- Aspen Pharmacare Holdings Limited
- AstraZeneca PLC
- Bayer AG
- Boehringer Ingelheim International GmbH
- Bristol-Myers Squibb Company
- Cipla Ltd.
- Dr. Reddy's Laboratories Ltd.
- F. Hoffmann-La Roche Ltd.
- GlaxoSmithKline plc
- Johnson & Johnson
- Lupin Limited
- Merck & Co., Inc.
- Mylan N.V.
- Novartis AG
- Pfizer Inc.
- Sanofi S.A.
- Sun Pharmaceutical Industries Ltd.
- Teva Pharmaceutical Industries Ltd.
Strategic Recommendations Empowering Industry Leaders to Navigate Market Complexities and Capitalize on Pleurodynia Treatment Opportunities
Industry leaders should prioritize investment in research and development to accelerate the pipeline of combination therapies that address both symptomatic relief and viral suppression. By forging collaborations with academic institutions and emerging biotech startups, organizations can gain early access to novel compounds and leverage complementary expertise. Additionally, establishing localized manufacturing hubs for active pharmaceutical ingredients and key excipients can mitigate tariff-related supply disruptions and support responsive scale-up.
Adopting digital health solutions is imperative for enhancing patient engagement and improving treatment adherence. Integrating teleconsultation platforms with connected injection devices and mobile applications will enable real-time monitoring of pain episodes and dosing patterns. These data streams, when analyzed through advanced analytics, can guide product enhancements and support value-based contracting discussions with payers.
Furthermore, building strategic partnerships across the distribution spectrum-ranging from hospital pharmacies to online retail and independent outlets-will ensure broad access to therapies. Engaging in value-based agreements that align pricing with demonstrated clinical outcomes can strengthen payer confidence and facilitate formulary inclusion. Investing in targeted patient support programs, including caregiver training for home administration and educational resources for specialty clinics, will further drive uptake and satisfaction.
By executing an integrated strategy that combines innovation, localized manufacturing, digital engagement, and collaborative commercial models, industry players can navigate market complexities effectively and position themselves at the forefront of pleurodynia treatment advancements.
Comprehensive Research Methodology Ensuring Robust Data Triangulation Accurate Market Mapping and Insightful Industry Analysis
The research methodology underpinning this analysis combined rigorous secondary research, primary qualitative interviews, and quantitative data validation to ensure comprehensive market insights. The secondary research phase entailed a thorough review of peer-reviewed journals, regulatory filings, and clinical trial registries to map existing treatments and emerging candidates. This foundational work established a detailed understanding of therapeutic mechanisms, formulation trends, and competitive activities.
Primary research involved in-depth interviews with key opinion leaders, including pulmonologists, infectious disease specialists, and pharmacists, to capture frontline perspectives on treatment efficacy, patient adherence challenges, and unmet needs. These interviews provided actionable insights into real-world clinical practices and validated assumptions derived from the literature review.
Quantitative validation was achieved through data triangulation, leveraging sales data from leading distributors, prescription trends across major healthcare markets, and patient outcome registries. A hybrid approach combined top-down analysis of macro-level drivers with bottom-up assessments of segment-specific dynamics, ensuring that the findings accurately reflect market realities.
Expert panel workshops were convened to test preliminary conclusions, refine segmentation logic, and stress-test strategic scenarios. The final deliverable integrates these multiple research streams into a cohesive framework, offering robust, data-driven insights to guide decision-making in the evolving pleurodynia treatment landscape.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Pleurodynia Treatment market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Pleurodynia Treatment Market, by Treatment Class
- Pleurodynia Treatment Market, by Dosage Form
- Pleurodynia Treatment Market, by Distribution Channel
- Pleurodynia Treatment Market, by End User
- Pleurodynia Treatment Market, by Region
- Pleurodynia Treatment Market, by Group
- Pleurodynia Treatment Market, by Country
- United States Pleurodynia Treatment Market
- China Pleurodynia Treatment Market
- Competitive Landscape
- List of Figures [Total: 16]
- List of Tables [Total: 2544 ]
Synthesizing Critical Findings to Illuminate the Current State and Future Trajectory of the Pleurodynia Treatment Landscape
In synthesizing the critical findings, it is evident that the pleurodynia treatment arena is at a transformative crossroads. The integration of targeted antivirals with established analgesic and antipyretic agents underscores a holistic approach to patient care, while innovative dosage forms are expanding access across diverse settings. Supply chain resilience, particularly in light of new tariff structures, has emerged as a strategic imperative, prompting investments in domestic production and regulatory engagement.
Segmentation insights reveal that treatment classes, dosage forms, distribution channels, and end-user endpoints collectively influence commercial strategies and patient outcomes. Tailored approaches that align product portfolios with specific clinical needs and administration preferences will drive differentiation in a competitive market. Regional nuances-from stringent standards in developed markets to surging demand in emerging economies-underscore the importance of localized strategies.
Key companies are demonstrating leadership through agile R&D collaborations, digital health integrations, and strategic manufacturing partnerships. As healthcare systems evolve toward value-based care, outcome-oriented agreements and patient support initiatives will be essential for demonstrating therapeutic impact and securing formulary access. For industry stakeholders, the convergence of scientific innovation, regulatory change, and shifting patient expectations presents an opportune moment to redefine pleurodynia care.
Looking forward, success will hinge on cohesive strategies that balance innovation with operational excellence. Companies that embrace cross-sector collaboration, leverage real-world evidence, and anticipate emerging market trends will be best positioned to deliver meaningful advances and improve patient quality of life.
Take Action Today to Engage with Associate Director Ketan Rohom and Secure Your In-Depth Pleurodynia Treatment Market Research Report
Don’t miss the opportunity to gain a competitive edge by accessing our comprehensive market research report on pleurodynia treatment. Reach out directly to Ketan Rohom, Associate Director, Sales & Marketing, to explore how our insights can support your strategic decision-making and drive growth. Engage today to secure tailored intelligence that will empower your organization to anticipate market shifts, optimize product development strategies, and capitalize on emerging opportunities.

- How big is the Pleurodynia Treatment Market?
- What is the Pleurodynia Treatment Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




