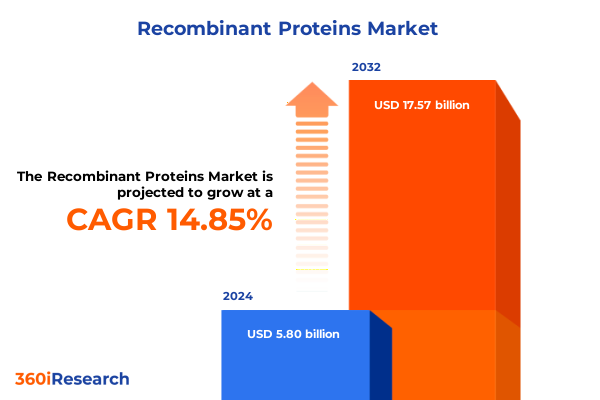
The Recombinant Proteins Market size was estimated at USD 6.65 billion in 2025 and expected to reach USD 7.62 billion in 2026, at a CAGR of 14.89% to reach USD 17.57 billion by 2032.
Unveiling the Pivotal Role of Recombinant Proteins in Driving Biotechnological Advancements and Therapeutic Breakthroughs Worldwide
Recombinant proteins have become the backbone of modern biotechnology, underpinning advancements in diagnostics, therapeutics and vaccine development that would have been inconceivable just a few decades ago. By leveraging genetic engineering to produce specific proteins in controlled host systems, researchers and manufacturers have unlocked unprecedented precision in drug design, disease detection and immunization strategies. The ability to tailor proteins with defined structural and functional attributes has revolutionized fields ranging from oncology to infectious disease, creating a new paradigm in how we approach health care and fundamental life science research.
The integration of recombinant technology into mainstream pharmaceutical and diagnostic workflows has driven substantial innovation in treatment modalities and laboratory techniques. With an expanding portfolio of recombinant enzymes, cytokines and growth factors, laboratories now conduct experiments with higher reproducibility and efficiency, while pharmaceutical companies develop biotherapeutics that offer improved safety profiles and targeted action mechanisms. As the pace of discovery accelerates, this report serves as an executive summary to distill the most critical trends, challenges and opportunities shaping the recombinant protein domain today.
Exploring Groundbreaking Technological and Regulatory Shifts Reshaping the Global Recombinant Protein Landscape Over the Last Decade
In recent years, the recombinant protein sector has experienced transformative shifts propelled by advances in expression technologies and heightened regulatory oversight. Cell-free synthesis, high-throughput screening platforms and the integration of bioinformatics have streamlined the development process, enabling rapid iteration and optimization of protein constructs. Simultaneously, regulatory agencies have harmonized quality and safety guidelines, reinforcing best practices for characterization, process validation and release criteria. These parallel movements have not only reduced time to market but have also elevated global standards, demanding ever-greater consistency and traceability throughout the production lifecycle.
Moreover, the convergence of gene editing tools such as CRISPR with traditional recombinant methods has facilitated the engineering of novel host cell lines, delivering higher yields and post-translational modifications tailored to therapeutic applications. Artificial intelligence and machine learning algorithms further enhance these capabilities by predicting expression outcomes and optimizing fermentation parameters. As a result, companies that harness these technological and regulatory advances are well positioned to deliver next-generation biologics faster, more efficiently and with a stronger assurance of regulatory compliance.
Assessing the Cumulative Influence of 2025 United States Tariff Policies on Supply Chains, Manufacturing Costs and Market Dynamics for Recombinant Proteins
The United States Tariff Act amendments implemented in early 2025 have exerted a cumulative impact on raw material costs and supply chain robustness for recombinant protein manufacturers. Import duties on specialized reagents, chromatography resins and cell culture media components have incrementally elevated production expenses, prompting organizations to reevaluate procurement strategies and supplier relationships. In turn, many producers have localized sourcing efforts or negotiated long-term supply agreements to mitigate price volatility and safeguard operational continuity.
These tariff adjustments have also accelerated the adoption of alternative materials and open-source protocols within research institutions and contract development organizations. Producers and end users are increasingly exploring in-house resin regeneration techniques, single-use technologies and bioprocess intensification methods to counterbalance external cost pressures. As a direct consequence, the landscape has shifted toward greater resilience and self-sufficiency, with market participants prioritizing vertical integration and strategic partnerships to ensure uninterrupted access to critical inputs.
Illuminating Core Segmentation Strategies Revealing Application Types, Product Categories, Expression Systems, End Users, Form Variations, and Channel Dynamics
The recombinant protein market exhibits multifaceted segmentation that drives tailored strategies for diverse stakeholders. By applications ranging from in vitro diagnostics and imaging agents in diagnostics to basic research, drug discovery and process development in research and development, each end of the spectrum demands proteins with distinct purity profiles and functional characteristics. The therapeutic segment spans autoimmune interventions, cardiovascular treatments and oncology therapies, while vaccine development encompasses both prophylactic inoculants and therapeutic vaccines, each with unique formulation and regulatory considerations.
When considering product type, cytokines, enzymes, growth factors and hormones serve specialized roles, necessitating differentiated production protocols and quality control measures. Expression systems further refine this landscape: bacterial platforms such as E. coli offer cost-effective, high-yield expression, whereas insect cells via baculovirus systems, mammalian hosts like CHO and HEK cells, transgenic plant models and yeast organisms such as Pichia pastoris and Saccharomyces cerevisiae introduce varying post-translational modifications. Form factors-from liquid formulations that simplify downstream processing to lyophilized preparations that enhance stability during storage-add another layer of complexity. Finally, sales channels including direct vendor engagements and distribution partnerships shape market access, while end users spanning academic and research institutes, contract research organizations, diagnostic laboratories and pharmaceutical and biotech companies, whether large pharma or emerging biotech firms, each navigate distinct procurement pathways and technical requirements.
This comprehensive research report categorizes the Recombinant Proteins market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Product Type
- Expression System
- Form
- Sales Channel
- Application
- End User
Comparative Regional Perspectives Highlighting Trends and Growth Drivers Across the Americas, EMEA and Asia-Pacific in Recombinant Protein Research and Bioproduction
Regional dynamics in the recombinant protein space underscore diverse drivers of innovation and adoption. In the Americas, a robust ecosystem of academic research hubs and contract development organizations fosters early-stage protein engineering and translational research. Access to venture capital and a supportive regulatory environment further accelerates pipeline advancement, making North America a critical launchpad for novel biologics and diagnostic reagents.
Across Europe, the Middle East and Africa, strong emphasis on biosimilar development and regulatory harmonization presents both opportunities and challenges. The European Medicines Agency’s evolving guidelines on comparability and quality standards have elevated expectations for protein characterization, spurring companies to invest in advanced analytical platforms. Meanwhile, emerging markets in the Middle East and Africa are increasingly forging public-private partnerships to build local manufacturing capabilities and reduce dependency on imports.
Asia-Pacific has emerged as a high-growth region driven by expanding biopharmaceutical manufacturing capacity, government incentives for biotechnology and a growing pool of skilled labor. Countries such as China, India and South Korea are rapidly scaling fermentation facilities and investing in next-generation expression systems, positioning the region as a major exporter of recombinant proteins and biosimilars worldwide.
This comprehensive research report examines key regions that drive the evolution of the Recombinant Proteins market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Profiling Leading Industry Stakeholders and Strategic Collaborations Driving Innovation, Competitive Positioning and Operational Excellence in the Recombinant Protein Sector
Within the competitive landscape, established companies and agile newcomers alike are shaping market dynamics through innovation and strategic partnerships. Leading life science tool providers continue to expand their portfolios of high-purity proteins, leveraging proprietary expression platforms and robust quality management systems to meet stringent regulatory requirements. Simultaneously, biopharmaceutical firms are collaborating with contract development and manufacturing organizations to accelerate clinical candidate progression and commercialization efforts, often entering co-development or licensing agreements to share risk and resources.
Mid-sized enterprises specialize in niche protein classes or novel expression technologies, differentiating through rapid customization services and digital process monitoring tools. Their focus on specialized applications, such as point-of-care diagnostics or personalized cancer vaccines, underscores the trend toward precision solutions. Strategic acquisitions and joint ventures are common, enabling companies to integrate complementary capabilities-from cell line engineering to downstream purification-thus streamlining their value chains and strengthening market positioning.
This comprehensive research report delivers an in-depth overview of the principal market players in the Recombinant Proteins market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Abcam plc
- Bio-Rad Laboratories, Inc.
- Bio-Techne Corporation
- Boehringer Ingelheim International GmbH
- Danaher Corporation
- Eli Lilly and Company
- F. Hoffmann-La Roche Ltd.
- Lonza Group Ltd.
- Merck KGaA
- Novo Nordisk A/S
- Sanofi S.A
- Sartorius AG
- Thermo Fisher Scientific Inc.
Strategic Recommendations for Industry Leaders to Enhance Production Efficiency, Regulatory Compliance and Innovation Pipelines Within the Recombinant Protein Industry
To thrive in the current recombinant protein landscape, industry leaders should prioritize investments in advanced expression systems that can deliver both high yields and tailored post-translational modifications, thereby meeting emerging therapeutic and diagnostic requirements. Diversifying supplier networks and establishing regional manufacturing hubs will provide resilience against future tariff changes and supply chain disruptions, while vertical integration of upstream and downstream processing can reduce lead times and improve cost predictability.
Engagement with regulatory authorities through early-stage consultation and participation in industry consortia will ensure alignment with evolving guidelines and streamline product approval timelines. Furthermore, integrating digital solutions such as data analytics, real-time process monitoring and predictive maintenance will enhance operational efficiency and support continuous improvement initiatives. Finally, fostering collaborative research partnerships with academic institutions and contract organizations will accelerate innovation and enable the rapid translation of discoveries into effective market-ready applications.
Outlining a Rigorous Research Methodology Integrating Primary Interviews, Secondary Data Analysis and Validation Processes for Comprehensive Recombinant Protein Insights
The findings presented in this report derive from a rigorous research methodology that combines comprehensive secondary data analysis with targeted primary interviews. Secondary sources included peer-reviewed journals, regulatory filings and patent databases to map technological developments and quality standards. These were complemented by primary engagements with industry executives, process development experts and end users to validate insights, capture emerging needs and uncover practical challenges in recombinant protein production.
A structured data triangulation process ensured the reliability of conclusions, cross-referencing quantitative metrics with qualitative feedback. The research team applied standardized frameworks to analyze segmentation dynamics, regional trends and competitive strategies, while adhering to stringent validation protocols. Throughout the project, methodological rigor was maintained by employing multiple layers of internal review and expert advisory input, guaranteeing that the final report reflects the most accurate and actionable intelligence available.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Recombinant Proteins market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Recombinant Proteins Market, by Product Type
- Recombinant Proteins Market, by Expression System
- Recombinant Proteins Market, by Form
- Recombinant Proteins Market, by Sales Channel
- Recombinant Proteins Market, by Application
- Recombinant Proteins Market, by End User
- Recombinant Proteins Market, by Region
- Recombinant Proteins Market, by Group
- Recombinant Proteins Market, by Country
- United States Recombinant Proteins Market
- China Recombinant Proteins Market
- Competitive Landscape
- List of Figures [Total: 18]
- List of Tables [Total: 2226 ]
Synthesizing Critical Findings to Highlight Strategic Imperatives, Knowledge Gaps and Future Trajectories in the Evolving Recombinant Protein Ecosystem
In synthesizing the critical insights from technological evolutions, tariff impacts, segmentation analyses and regional dynamics, it becomes evident that the recombinant protein industry stands at a crossroads of opportunity and challenge. Organizations that effectively harness cutting-edge expression platforms, adhere to evolving regulatory standards and cultivate resilient supply chains will secure sustainable competitive advantages. Equally, those that leverage strategic partnerships and digital capabilities will accelerate their path to market and respond more swiftly to emerging scientific demands.
Looking ahead, the continued convergence of biotechnological innovation and data-driven process optimization promises to unlock new frontiers in personalized medicine, rapid diagnostics and vaccine development. To capitalized on these trajectories, stakeholders must remain agile, continuously refining their operational models and collaboration strategies. By building on the insights outlined in this summary, decision-makers can chart a robust trajectory for growth and innovation within the dynamic recombinant protein ecosystem.
Empower Your Decision-Making by Connecting with Ketan Rohom, Associate Director of Sales & Marketing, to Secure Exclusive Access to the Comprehensive Market Research Report
To gain unparalleled clarity on the evolving recombinant proteins market and to make informed strategic decisions, reaching out to Ketan Rohom, Associate Director of Sales & Marketing, will connect you to the full depth of our comprehensive market intelligence report. Each chapter of the report has been meticulously crafted to cover technological innovations, regulatory developments, segmentation dynamics and regional nuances, ensuring that you receive actionable insights tailored to your organization’s objectives. By engaging directly with Ketan, you will benefit from a personalized briefing that highlights the sections most relevant to your strategic needs, whether you are refining product pipelines or exploring new geographic markets.
Contacting Ketan opens the door to exclusive add-on data sets and bespoke consulting support designed to accelerate your market entry and growth plans. His expertise in synthesizing complex industry trends into clear recommendations will streamline your evaluation process and equip your team with the evidence-based guidance required to outpace competitors. Don’t miss the opportunity to fortify your strategic roadmap with the latest and most reliable intelligence-initiate your outreach today and elevate your decision-making with insights that matter.

- How big is the Recombinant Proteins Market?
- What is the Recombinant Proteins Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




