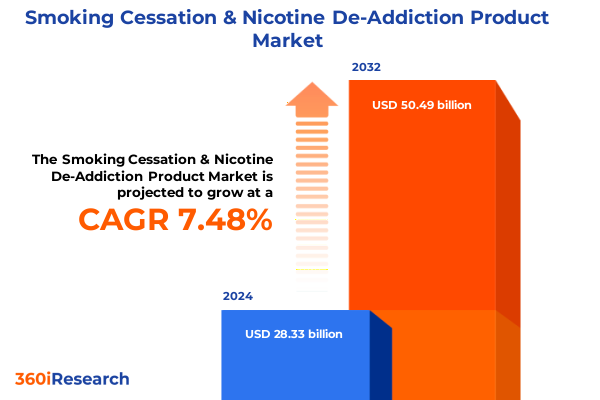
The Smoking Cessation & Nicotine De-Addiction Product Market size was estimated at USD 30.31 billion in 2025 and expected to reach USD 32.49 billion in 2026, at a CAGR of 7.55% to reach USD 50.49 billion by 2032.
Introducing the Comprehensive Landscape of Smoking Cessation and Nicotine De-Addiction Products to Establish Strategic Foundations for Stakeholders
An effective introduction lays the groundwork for understanding the multifaceted smoking cessation and nicotine de-addiction product ecosystem that has rapidly evolved over recent years. Against a backdrop of heightened regulatory scrutiny, shifting consumer preferences, and novel technological interventions, stakeholders require a holistic narrative that captures the interplay between clinical efficacy, behavioral support, and market forces. In addition, the growing convergence of digital health solutions and personalized medicine underscores the need to evaluate not only traditional therapies but also emerging modes of intervention that bridge pharmacological and psychosocial approaches.
This overview aims to orient decision-makers by highlighting the critical components of the landscape, spanning behavioral therapy modalities such as group-based counseling and individualized support to a spectrum of nicotine replacement products encompassing gum, patches, inhalers, lozenges, and nasal sprays. Moreover, it considers the expanded portfolio of prescription agents, including bupropion, varenicline, and cytisine. Through this lens, the introduction sets the stage for exploring transformative shifts, tariff-driven disruptions, and strategic segmentation insights. Consequently, readers will be equipped with a clear framework for navigating the complexities of product development, regulatory compliance, and consumer engagement in the smoke-free pursuit.
Exploring the Major Transformational Shifts Redefining Smoking Cessation and Nicotine Replacement Therapies across Regulatory, Technological, and Consumer Trends
The smoking cessation market has witnessed profound transformations driven by breakthroughs in digital therapeutics, heightened focus on harm reduction, and evolving reimbursement models. Furthermore, accelerated by advances in telehealth platforms, behavioral therapies have transcended the confines of in-person sessions to offer remote group and one-on-one counseling, enabling greater accessibility and adherence. Concurrently, the integration of mobile applications and wearable biosensors has introduced real-time monitoring of cravings and personalized feedback loops, which enhance the efficacy of traditional nicotine replacement therapies.
Moreover, regulatory agencies worldwide are increasingly mandating standardized clinical outcomes and mandating transparent labeling for nicotine strength, propelling manufacturers to reformulate products for optimized safety and effectiveness. At the same time, consumer attitudes have shifted markedly toward harm reduction; this has spurred innovation in alternative nicotine delivery systems that balance user satisfaction with lower toxicant profiles. Consequently, stakeholders must reconcile the tension between established pharmacotherapies and disruptive modalities, positioning their pipelines to capture emerging segments while maintaining compliance with tightening safety and efficacy standards.
Assessing the Comprehensive Effects of Newly Imposed US Tariffs in 2025 on Smoking Cessation Product Sourcing, Pricing Dynamics, and Industry Profit Margins
The introduction of new United States tariffs in 2025 on imported nicotine-based products has significantly impacted the cost structure and supply chain dynamics of smoking cessation portfolios. Specifically, additional duties on raw nicotine extracts and finished nicotine replacement packaging materials have elevated landed costs for manufacturers reliant on offshore production. As a result, pricing strategies for over-the-counter products such as patches, gum, and lozenges have come under pressure, compelling companies to reassess sourcing from domestic suppliers or to absorb incremental expenses to maintain competitive retail pricing.
Furthermore, prescription drugs like varenicline and bupropion, which often depend on active pharmaceutical ingredients sourced internationally, have experienced margin compression. This shift has catalyzed partnerships between pharmaceutical firms and domestic API manufacturers to mitigate exposure to cross-border levies. In addition, inventors of inhalable and nasal spray devices have had to revisit design for manufacturability principles to incorporate locally available components. The cumulative effect of the 2025 tariff regime thus underscores the strategic imperative of supply chain resiliency, vertical integration, and agile product reengineering to safeguard profitability and preserve market share.
Uncovering Deep Insights from Behavioral Therapies to Prescription Drugs through Multi-Dimensional Segmentation in the Smoking Cessation Market
A nuanced understanding of the smoking cessation market emerges when evaluating product type, route of administration, distribution channel, end-user demographics, and nicotine strength. Examining behavioral therapy, for instance, reveals how group counseling sessions foster peer support dynamics, while individual counseling delivers tailored interventions for high-risk segments. Within nicotine replacement therapies, differences between gum, lozenges, patches, inhalers, and nasal sprays reflect varying onset times and user preferences, shaping adherence and satisfaction.
When considering administration routes, the choice between oral lozenges and sublingual tablets or between inhalation devices and transdermal patches determines both pharmacokinetic profiles and user convenience. The evolution of online pharmacies alongside brick-and-mortar pharmacies broadens access channels, allowing products to reach tech-savvy adults seeking discreet delivery, while also serving pregnant women and adolescents through regulated frameworks. Moreover, differentiating heavy smokers from low-consumption users necessitates adjustments in nicotine concentration across low, medium, and high-strength formulations to meet dependency levels without compromising safety. Through these interwoven segmentation lenses, companies can craft targeted value propositions that resonate with distinct consumer cohorts and optimize market penetration across diverse touchpoints.
This comprehensive research report categorizes the Smoking Cessation & Nicotine De-Addiction Product market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Product Type
- Route Of Administration
- Level Of Dependence
- Distribution Channel
- Customer Type
Highlighting Key Regional Dynamics Shaping the Trajectory of Smoking Cessation and Nicotine De-Addiction Solutions across Major Global Markets
Regional dynamics continue to shape the trajectory of smoking cessation and nicotine de-addiction solutions, reflecting diverse regulatory environments, cultural attitudes toward tobacco, and healthcare infrastructure maturity. In the Americas, strong public health advocacy and widespread insurance coverage have bolstered adoption of both behavioral interventions and pharmacy-dispensed nicotine replacement products. This robust ecosystem has also fostered strategic alliances between digital health start-ups and established pharmaceutical companies, accelerating the rollout of app-based cessation platforms.
Conversely, Europe, the Middle East, and Africa demonstrate heterogeneous market conditions; Western European nations emphasize strict product labeling and reimbursement frameworks, while emerging markets in the Middle East and Africa are experiencing nascent demand growth fueled by rising awareness campaigns and policy shifts to curb smoking prevalence. In the Asia-Pacific region, rapid urbanization and expanding telemedicine infrastructure are driving increased uptake of remote counseling services and nicotine patches, even as regulatory approvals for prescription drugs differ widely across countries. These regional variations underline the necessity for localized go-to-market approaches that balance global best practices with culturally attuned engagement strategies.
This comprehensive research report examines key regions that drive the evolution of the Smoking Cessation & Nicotine De-Addiction Product market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Profiling Leading Innovators and Established Pharmaceutical Players Driving Advancements and Competitive Differentiation in Smoking Cessation Offerings
The competitive landscape is anchored by pharmaceutical giants and nimble innovators working in tandem to expand therapeutic options. Leading multinational corporations have leveraged extensive R&D capabilities to refine second-generation nicotine replacement devices and to secure approvals for novel prescription compounds targeting neural receptor pathways. Meanwhile, specialized biotech ventures are exploring cytisine-based therapies, capitalizing on lower production costs and simplified regulatory pathways in select markets.
Strategic partnerships have become commonplace, with digital therapeutics firms collaborating with established drug manufacturers to integrate behavioral modules into inhaler or patch packaging. This convergence underscores a broader trend toward end-to-end cessation solutions that combine pharmacology with data-driven coaching. Furthermore, contract manufacturing organizations are playing a pivotal role by offering flexible production runs and rapid scale-up capabilities, enabling both established and emerging players to respond swiftly to tariff-induced supply disruptions. These synergistic relationships are redefining value chains, promoting innovation, and intensifying competition across the product spectrum.
This comprehensive research report delivers an in-depth overview of the principal market players in the Smoking Cessation & Nicotine De-Addiction Product market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- 22nd Century Group, Inc.
- Achieve Life Sciences, Inc.
- Alkalon A/S
- Axsome Therapeutics, Inc.
- British American Tobacco PLC
- Cipla Ltd.
- Ditch Labs
- Dr. Reddy’s Laboratories Limited
- Enorama Pharma AB
- Evotec SE
- GlaxoSmithKline PLC
- Glenmark Pharmaceuticals Limited
- Haleon Group of Companies
- Imperial Tobacco Company of India Limited
- Itaconix by Revolymer
- Japan Tobacco Inc.
- JOYSBIO (Tianjin) Biotechnology Co., Ltd
- Kenvue Inc.
- KONTAM TECH COMPANY
- MAG FLARE(MACAO)TECHNOLOGY LIMITED
- Novartis International AG
- Perrigo Company PLC
- Pharmastrat Ltd.
- Pierre Fabre S.A.
- Pivot Health Technologies, Inc.
- Provide Community Interest Company
- Reed Wellbeing Limited
- Rusan Pharma Ltd.
- Samyang Holdings Corporation
- Shenzhen InterEar Intelligent Technology Co., Ltd.
- Smoke Free
- Smokefree Hampshire
- Soar Biotech Co.,Ltd
- Sparsha Pharma International Pvt. Ltd.
- Strides Pharma Science Limited
- ZYROGUM
Outlining Targeted, Actionable Recommendations to Empower Industry Leaders in Driving Growth and Innovation within the Smoking Cessation Ecosystem
Industry leaders should prioritize the development of integrated delivery systems that align pharmacological efficacy with seamless user experiences. By investing in modular device platforms, companies can address multiple nicotine strengths and administration routes without incurring separate manufacturing lines. Moreover, fostering strategic collaborations between pharma and digital health providers will enable the creation of robust cessation ecosystems, where app-based behavioral coaching complements clinical therapies to boost long-term abstinence rates.
Additionally, establishing nearshore or onshore manufacturing capabilities can insulate supply chains from tariff volatility and enhance responsiveness to regulatory changes. Pursuing joint ventures with domestic API suppliers may further reduce exposure to import duties. Equally important is the adoption of dynamic pricing models that reflect regional purchasing power while preserving margin thresholds. In parallel, firms must engage proactively with regulatory bodies to shape emerging guidelines on product labeling, safety standards, and telehealth integration. These measures collectively will position industry leaders to capture evolving market opportunities and deliver superior value to end-users.
Detailing Robust Research Methodology Employed to Deliver Reliable, Transparent Insights into the Smoking Cessation and Nicotine De-Addiction Sector
Our research process combined primary and secondary methodologies to ensure comprehensive, data-driven insights. We conducted in-depth interviews with healthcare professionals, regulatory experts, and product development leaders to gather nuanced perspectives on emerging treatment paradigms. These qualitative inputs were complemented by an extensive review of peer-reviewed journals, government policy documents, and clinical trial registries to validate efficacy benchmarks and safety profiles across product categories.
Furthermore, we employed advanced data analytics techniques to synthesize real-world usage patterns drawn from prescription drug databases and over-the-counter sales reports. Supply chain analyses incorporated tariff schedules and manufacturing cost indices to model the financial impact of import levies. Our multi-tiered approach, which integrates both macroeconomic trends and micro-level consumer behaviors, ensures that the study’s findings are robust, transparent, and actionable. Quality control measures, including cross-validation by independent experts, underpin the credibility of the insights presented throughout this report.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Smoking Cessation & Nicotine De-Addiction Product market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Smoking Cessation & Nicotine De-Addiction Product Market, by Product Type
- Smoking Cessation & Nicotine De-Addiction Product Market, by Route Of Administration
- Smoking Cessation & Nicotine De-Addiction Product Market, by Level Of Dependence
- Smoking Cessation & Nicotine De-Addiction Product Market, by Distribution Channel
- Smoking Cessation & Nicotine De-Addiction Product Market, by Customer Type
- Smoking Cessation & Nicotine De-Addiction Product Market, by Region
- Smoking Cessation & Nicotine De-Addiction Product Market, by Group
- Smoking Cessation & Nicotine De-Addiction Product Market, by Country
- United States Smoking Cessation & Nicotine De-Addiction Product Market
- China Smoking Cessation & Nicotine De-Addiction Product Market
- Competitive Landscape
- List of Figures [Total: 17]
- List of Tables [Total: 2862 ]
Concluding Strategic Takeaways and Critical Insights to Guide Stakeholders toward Effective Decision-Making in Smoking Cessation Product Development
In conclusion, the smoking cessation and nicotine de-addiction landscape is undergoing a period of dynamic transformation, driven by regulatory tightening, technological innovation, and shifting consumer preferences. Stakeholders who embrace integrated treatment models-combining behavioral therapies with optimized pharmacological delivery systems-are best positioned to capture emerging market growth. Simultaneously, proactive supply chain management and strategic alliances will be critical to mitigating the financial impacts of tariff impositions and regulatory headwinds.
As companies refine their product portfolios, the insights highlighted herein provide a clear roadmap for segmentation-driven targeting, regional expansion tailored to local market conditions, and partnership-driven innovation. By synthesizing these strategic imperatives, decision-makers can navigate uncertainty, accelerate product development, and achieve sustainable competitive advantage. Ultimately, organizations that translate these lessons into decisive action will lead the charge in helping millions of users achieve lasting smoking cessation.
Engage with Ketan Rohom to Secure Comprehensive Market Intelligence and Unlock Strategic Advantages in the Smoking Cessation Product Landscape
To explore how our in-depth market research can sharpen your competitive edge in the rapidly evolving smoking cessation arena, engage directly with Ketan Rohom, Associate Director of Sales & Marketing at 360iResearch. Ketan brings a wealth of experience guiding decision-makers through complex market landscapes and can tailor insights to your specific strategic objectives. By initiating this dialogue, you will gain personalized support in accessing comprehensive data, uncovering hidden opportunities, and accelerating product innovation timelines.
Ketan will walk you through the report’s unique value propositions, including segmentation analytics, tariff impact assessments, and regional dynamics that drive growth. He will also explain how our proprietary methodology ensures data integrity and relevance. This conversation will help you align your investment priorities with market realities and confidently navigate emerging regulatory challenges.
Reach out to schedule a consultation and learn how these insights can translate into actionable milestones for your organization. Partnering with Ketan will empower your team to make informed decisions, optimize resource allocation, and secure a robust market position. Don’t miss this opportunity to gain a strategic advantage and steer your product portfolio toward sustainable success.

- How big is the Smoking Cessation & Nicotine De-Addiction Product Market?
- What is the Smoking Cessation & Nicotine De-Addiction Product Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




