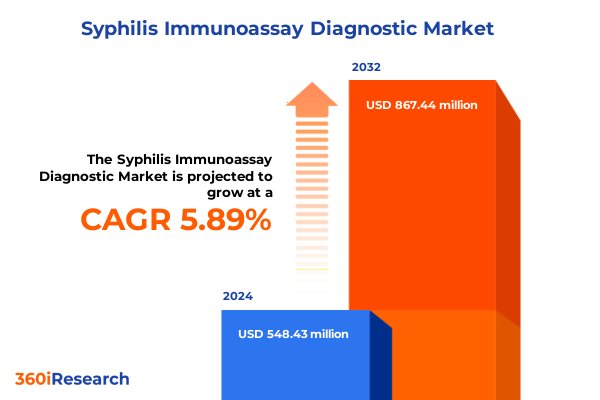
The Syphilis Immunoassay Diagnostic Market size was estimated at USD 578.43 million in 2025 and expected to reach USD 611.81 million in 2026, at a CAGR of 5.95% to reach USD 867.44 million by 2032.
Setting the Stage for Evolution in Syphilis Immunoassay Diagnostics Amid Rising Prevalence and Technological Breakthroughs to Enhance Early Detection
In recent years, the resurgence of syphilis has posed significant challenges for public health authorities worldwide. As incidence rates continue to climb, particularly in vulnerable populations, the demand for robust diagnostic solutions has never been greater. Immunoassay diagnostics, with their high sensitivity and specificity, have emerged as indispensable tools in the early detection and management of syphilis infections. By enabling rapid and accurate screening, these assays contribute to timely therapeutic interventions, thereby reducing transmission and improving patient outcomes.
Within this evolving landscape, a wide array of diagnostic formats has been deployed, ranging from fully automated laboratory analyzers to portable point-of-care devices. Automated analyzers deliver high throughput and standardized performance, catering to centralized laboratories processing large sample volumes. In contrast, semi-automated systems offer a balance between automation and operator control, while point-of-care platforms bring testing closer to the patient, accelerating diagnosis in clinical settings and outreach programs. Each modality plays a unique role in addressing regional testing needs, infrastructure constraints, and healthcare priorities.
Regulatory agencies have also recognized the critical role of reliable diagnostics in combating syphilis. Harmonized guidelines and accelerated approval pathways are incentivizing assay developers to pursue innovation in assay design and performance validation. As stakeholders operate under evolving regulatory landscapes, alignment with quality standards and rigorous clinical evaluation becomes a competitive differentiator, driving the next wave of immunoassay advancements.
As healthcare stakeholders seek to optimize diagnostic workflows and public health strategies, understanding the nuances of immunoassay technologies, supply chain dynamics, and regulatory frameworks is essential. This executive summary provides a coherent foundation, highlighting key trends, market shifts, and strategic imperatives within the syphilis immunoassay diagnostic domain. By laying out this landscape, decision-makers can better navigate emerging opportunities and challenges, driving innovation and ultimately improving the quality of care for affected populations.
Navigating Technological and Epidemiological Transformations Reshaping the Syphilis Immunoassay Diagnostic Space to Enhance Clinical Efficacy and Patient Outcomes
In the last decade, the syphilis immunoassay diagnostic sector has undergone transformative shifts driven by technological innovation and changing epidemiological patterns. Advanced assay platforms now integrate chemiluminescent and fluorescence detection modalities, offering lower detection thresholds and enhanced precision. The incorporation of digital connectivity and cloud-based data management into automated analyzers enables seamless integration with laboratory information systems, facilitating real-time result reporting and analytics. Concurrently, miniaturized point-of-care devices leverage microfluidics and cartridge-based architectures to deliver rapid, on-site testing without compromising analytical performance.
Parallel to technological progress, epidemiological shifts have reshaped testing priorities. A notable resurgence of syphilis among key populations, coupled with rising rates of congenital transmission, has spurred public health initiatives to deploy more widespread and accessible screening programs. These efforts have catalyzed demand for flexible diagnostic solutions capable of high throughput in centralized settings and portable formats in field conditions. Moreover, heightened awareness and targeted funding have encouraged assay developers to refine assay chemistries and optimize sample volume requirements for diverse specimen types.
Regulatory landscapes have also evolved, with agencies endorsing streamlined approval pathways for novel immunoassay technologies. Quality benchmarks emphasizing clinical sensitivity and specificity have become more stringent, prompting manufacturers to invest in rigorous performance validation and continuous improvement. As a result, the market is witnessing a convergence of high-precision automated platforms with agile decentralized testing options, fundamentally reshaping how syphilis diagnoses are conducted and integrated into patient care pathways.
Assessing the Multifaceted Impact of 2025 United States Tariffs on Syphilis Immunoassay Diagnostic Supply Chains, Cost Structures and Procurement Dynamics
In 2025, the United States implemented a series of tariffs affecting imported diagnostic components, with significant ramifications for the syphilis immunoassay sector. These measures targeted raw reagents, specialized assay kits and key instrument components, leading to increased landed costs and supply chain realignments. Diagnostic manufacturers and laboratories have responded by diversifying their sourcing strategies, exploring alternative suppliers, and in some cases accelerating domestic production of critical reagents. The cumulative effect of these tariffs has extended lead times for kit deliveries and prompted cost-containment measures across the supply chain.
Laboratories reliant on imported immunoassay reagents now face upward pressure on per-test expenses, compelling procurement teams to renegotiate contracts and consolidate supplier portfolios. Instrument vendors have offset some tariff impacts by localizing assembly operations and optimizing component designs to reduce reliance on tariff-ed imports. At the same time, distributors have had to adjust inventory management and pricing models to accommodate fluctuating duty schedules, influencing adoption rates in price-sensitive healthcare settings.
Despite these challenges, the sector has demonstrated resilience through innovation in reagent formulations and strategic partnerships. Efforts to streamline assay workflows, such as lyophilized reagents and modular analyzer architectures, have mitigated cost volatility. Additionally, collaborative initiatives between industry associations and regulatory bodies are underway to harmonize tariff classifications and minimize compliance uncertainties. Collectively, these adaptive strategies underscore the sector’s capacity to navigate trade policy shifts while maintaining the integrity and availability of syphilis immunoassay diagnostics.
Uncovering Critical Insights Across Product Type, Technology, Sample Type and End User Segments Driving the Syphilis Immunoassay Diagnostic Market
Deep segmentation analysis reveals critical nuances shaping the syphilis immunoassay diagnostic market. By product type, the landscape spans a spectrum from instrument platforms to kit-based reagents and software solutions. Automated analyzers cater to high-volume laboratories seeking maximal throughput, while point-of-care devices deliver agility for decentralized testing environments. Semi-automated systems bridge these extremes, offering balance between manual operations and full automation. Within the kits domain, control kits ensure assay accuracy and internal calibration, ready-to-use kits simplify workflow for rapid deployment, and reagent kits supply the raw assay components enabling customization by end users.
Technological segmentation underscores the prominence of chemiluminescence immunoassays, which deliver exceptional sensitivity and dynamic range. Enzyme-linked immunosorbent assays maintain broad adoption due to established performance records and cost efficiencies, whereas fluorescence immunoassays are gaining traction for their capacity to multiplex and detect low-abundance biomarkers. Sample type considerations further differentiate offerings, with serum and plasma samples dominating centralized laboratory workflows and whole blood assays proving vital for point-of-care and field applications.
End-user segmentation highlights the diverse spectrum of testing environments. Blood banks emphasize high-volume screening to ensure transfusion safety, clinics require rapid turnaround for patient management, diagnostic laboratories prioritize standardized processes and data integration, and hospitals integrate immunoassay testing into broader clinical decision support systems. Understanding these interrelated segments provides a comprehensive view of where innovation and investment can deliver the greatest impact within the syphilis immunoassay diagnostic ecosystem.
This comprehensive research report categorizes the Syphilis Immunoassay Diagnostic market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Product Type
- Technology
- Sample Type
- End User
Exploring Regional Dynamics in the Americas, Europe Middle East & Africa and Asia-Pacific Underpinning Syphilis Immunoassay Diagnostic Developments
Regional dynamics are pivotal in shaping the development and adoption of syphilis immunoassay diagnostics. In the Americas, robust public health infrastructure and established reimbursement frameworks have driven widespread screening programs, with the United States leading initiatives for prenatal and high-risk population testing. Blood banks and diagnostic laboratories in North America leverage advanced automated analyzers, while community clinics in Latin America increasingly adopt point-of-care solutions to bridge access gaps in remote areas.
In Europe, Middle East and Africa, regulatory harmonization efforts such as the CE marking process have streamlined market entry for novel platforms. European healthcare systems emphasize cost-effectiveness and integrated diagnostic networks, fostering partnerships between governments and manufacturers to deploy high-throughput laboratory workflows. In the Middle East and Africa, varying levels of healthcare infrastructure necessitate a dual focus on portable assay platforms for rural outreach and centralized analyzers in urban tertiary centers, underscoring the need for adaptable solution portfolios.
Across Asia-Pacific, emerging economies are witnessing accelerated investment in diagnostic capacity building. Governments in countries like India, China and Australia are expanding national screening initiatives, driving demand for both conventional enzyme-linked immunosorbent assays and newer chemiluminescence platforms. The proliferation of local manufacturing capabilities is also reducing dependence on imports, enhancing supply chain resilience. These regional profiles highlight the importance of tailored strategies that align product offerings, distribution models and stakeholder partnerships to address distinct market dynamics and public health priorities.
This comprehensive research report examines key regions that drive the evolution of the Syphilis Immunoassay Diagnostic market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Profiling Leading Innovators and Strategic Collaborators Shaping the Competitive Landscape of the Syphilis Immunoassay Diagnostic Sector
Leading companies are actively shaping the competitive landscape of syphilis immunoassay diagnostics through strategic investments in research and development, collaborations and portfolio diversification. Major diagnostics conglomerates have expanded their assay pipelines to include next-generation chemiluminescence and fluorescence platforms, while strategically acquiring niche players to strengthen their reagent kit offerings. At the same time, specialized biotech firms are leveraging advances in protein engineering and nanotechnology to develop ultrasensitive assay components that reduce sample volumes and expedite turnaround times.
Strategic collaborations between instrument manufacturers and reagent developers have enabled seamless integration of hardware and assay chemistries, enhancing overall system performance and facilitating regulatory approvals. Software providers are also partnering with laboratory information management system vendors to offer analytics-driven insights, enabling laboratories to optimize testing workflows and maintain quality compliance. In parallel, emerging startups are targeting underserved markets with portable point-of-care devices designed for ease of use and minimal infrastructure requirements.
These competitive dynamics underscore a trend toward modular, interoperable diagnostic ecosystems where assay chemistries, instrumentation and software converge. Companies that can demonstrate scalability, regulatory expertise and a clear value proposition for diverse end users are positioning themselves as leaders. Moreover, ongoing investments in clinical validation studies and real-world performance data are becoming essential differentiators, bolstering market credibility and fostering long-term partnerships with healthcare stakeholders.
This comprehensive research report delivers an in-depth overview of the principal market players in the Syphilis Immunoassay Diagnostic market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Abbott Laboratories
- Beckman Coulter, Inc.
- Becton Dickinson and Company
- Bio-Rad Laboratories, Inc.
- bioMérieux SA
- bioMérieux SA
- F. Hoffmann-La Roche Ltd
- Ortho Clinical Diagnostics, LLC
- Siemens Healthineers AG
- Thermo Fisher Scientific Inc.
- Trinity Biotech plc
Delivering Pragmatic Strategies and Roadmaps for Industry Stakeholders to Capitalize on Growth Opportunities in Syphilis Immunoassay Diagnostics
As the syphilis immunoassay diagnostic market continues to evolve, industry leaders must adopt proactive strategies to capitalize on emerging opportunities and mitigate potential risks. Investing in the development of point-of-care platforms will address the growing need for decentralized testing in both urban clinics and remote settings. At the same time, optimizing automated analyzer workflows through enhanced software integration and predictive maintenance capabilities can drive laboratory efficiency and reduce total cost of ownership.
Stakeholders should also prioritize supply chain diversification by establishing partnerships with multiple reagent suppliers, including domestic manufacturers, to buffer against tariff-induced cost fluctuations and delivery delays. Engaging with regulatory authorities early in the product development cycle can expedite approvals and ensure compliance with evolving quality standards. Additionally, fostering collaborative networks with public health agencies, non-governmental organizations and academic institutions can enhance market access and support targeted screening initiatives.
Finally, companies should leverage digital health solutions, such as cloud-based data analytics and telemedicine integrations, to provide end-to-end diagnostic services. By combining assay innovations with data-driven insights, organizations can offer value-added services that improve clinical decision-making and patient outcomes. Embracing these actionable recommendations will not only strengthen competitive positioning but also advance public health objectives by facilitating timely and accurate syphilis diagnostics.
Detailing Rigorous Methodological Framework Combining Qualitative and Quantitative Approaches to Deliver Robust Insights on Syphilis Immunoassay Diagnostics
In conducting this analysis, a rigorous methodological framework was employed to ensure the integrity and validity of insights. Primary research comprised in-depth interviews with key opinion leaders, including laboratory directors, infectious disease specialists and procurement executives, to gather firsthand perspectives on diagnostic trends, operational challenges and strategic priorities.
Secondary research involved a comprehensive review of peer-reviewed journals, regulatory guidelines, clinical white papers and public health reports to contextualize market developments and validate technical specifications. Proprietary databases were leveraged to track patent filings, technology licenses and competitive dynamics, while industry publications provided intelligence on strategic collaborations and supply chain movements.
The research process incorporated both qualitative and quantitative techniques. Qualitative coding was applied to interview transcripts, enabling thematic analysis of stakeholder feedback. Quantitative analyses included mapping of product pipelines, evaluation of diagnostic performance metrics and assessment of pricing structures. Triangulation methods were used to cross-verify data points across multiple sources, ensuring consistency and reducing the risk of bias.
Data validation protocols involved systematic checks against publicly available disclosures and direct verification with manufacturer representatives. This methodological rigor underpins the credibility of the findings presented herein, providing stakeholders with a robust foundation for strategic decision-making in the dynamic syphilis immunoassay diagnostic landscape.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Syphilis Immunoassay Diagnostic market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Syphilis Immunoassay Diagnostic Market, by Product Type
- Syphilis Immunoassay Diagnostic Market, by Technology
- Syphilis Immunoassay Diagnostic Market, by Sample Type
- Syphilis Immunoassay Diagnostic Market, by End User
- Syphilis Immunoassay Diagnostic Market, by Region
- Syphilis Immunoassay Diagnostic Market, by Group
- Syphilis Immunoassay Diagnostic Market, by Country
- United States Syphilis Immunoassay Diagnostic Market
- China Syphilis Immunoassay Diagnostic Market
- Competitive Landscape
- List of Figures [Total: 16]
- List of Tables [Total: 1113 ]
Synthesizing Critical Findings and Strategic Imperatives to Inform Decision Making in the Evolving Syphilis Immunoassay Diagnostic Ecosystem
This executive summary has synthesized critical insights into the evolving landscape of syphilis immunoassay diagnostics, highlighting the intersection of technological advancements, regulatory developments and trade policy impacts. Key trends include the shift toward integrated automated and point-of-care platforms, the adoption of advanced detection modalities such as chemiluminescence and fluorescence, and the strategic responses to tariffs affecting supply chain resilience.
Segmentation analyses have elucidated the diverse requirements of laboratory instruments, kit types and software solutions, as well as the unique demands across blood banks, clinics, diagnostic laboratories and hospitals. Regional profiling underscores the importance of tailoring strategies to the Americas, Europe Middle East & Africa and Asia-Pacific, where healthcare infrastructure, regulatory regimes and public health priorities vary significantly.
Competitive dynamics reveal that companies capable of delivering modular, interoperable diagnostic ecosystems, supported by robust clinical validation and digital health integration, will lead market growth. Actionable recommendations, including investment in decentralized testing, supply chain diversification and regulatory engagement, provide a clear roadmap for stakeholders seeking to enhance their strategic positioning.
Ultimately, the syphilis immunoassay diagnostic ecosystem is at a pivotal juncture, with opportunities for innovation and collaboration poised to improve disease surveillance, patient outcomes and healthcare efficiency. This summary offers a consolidated view of market drivers and strategic imperatives, equipping decision-makers with the insights needed to navigate upcoming challenges and capitalize on new growth avenues.
Engage with Ketan Rohom Associate Director of Sales & Marketing to Access the Comprehensive Syphilis Immunoassay Diagnostic Market Research Report Today
To explore these comprehensive insights in detail and gain a competitive edge in the syphilis immunoassay diagnostic sector, we invite you to connect with Ketan Rohom Associate Director of Sales & Marketing. By securing this in-depth market research report, your organization will access a granular analysis of technological trends, regulatory considerations, segmentation dynamics and regional profiles. Armed with these actionable intelligence and strategic recommendations, you will be positioned to drive innovation, optimize procurement strategies and enhance patient care. Reach out today to purchase the full report and empower your team with the knowledge required to navigate the evolving diagnostic landscape effectively. Ensure your organization stays ahead of market disruptions and maximizes emerging opportunities by leveraging the expertise contained within this essential report.

- How big is the Syphilis Immunoassay Diagnostic Market?
- What is the Syphilis Immunoassay Diagnostic Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




