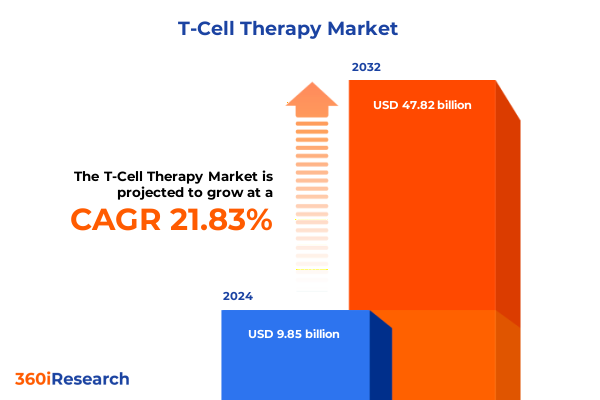
The T-Cell Therapy Market size was estimated at USD 12.03 billion in 2025 and expected to reach USD 14.69 billion in 2026, at a CAGR of 21.79% to reach USD 47.82 billion by 2032.
Exploring the Transformative Potential of T-Cell Therapy in Revolutionizing Treatment Paradigms across Oncology, Autoimmune, and Infectious Diseases
The advent of T-cell therapy has redefined the limits of modern medicine by harnessing the body’s own immune cells to target and eliminate disease. Through pioneering techniques in adaptive immunotherapy, researchers and clinicians collaborate to engineer patient-derived or donor-derived T cells capable of recognizing and destroying pathological cells with unprecedented precision. This paradigm shift promises not only new treatment avenues for malignancies but also novel approaches to managing chronic inflammatory and infectious conditions. With each milestone in preclinical validation and clinical translation, the potential of T-cell therapy to deliver durable remissions and improve patient outcomes has become increasingly evident, capturing the attention of biopharma sponsors, healthcare providers, and regulatory bodies alike.
As the complexity of cell engineering has grown, so too has the sophistication of manufacturing processes, regulatory frameworks, and delivery models. Today’s landscape reflects a maturation from laboratory benches to specialized clinical settings equipped to support complex cell handling and infusion procedures. Early adopters continue to refine patient eligibility criteria, dosing regimens, and safety monitoring protocols to maximize therapeutic benefit and minimize adverse events. In parallel, partnerships between academic institutions, contract developers, and in-house manufacturing facilities facilitate scalability and broaden access to cutting-edge therapies. As a result, the foundation for sustained innovation in T-cell therapy has been firmly established, setting the stage for transformational advances in the years to come.
Navigating Landmark Technological Advances and Regulatory Milestones Redefining the Landscape of T-Cell Immunotherapy Development
Over the past decade, landmark scientific breakthroughs and regulatory endorsements have propelled T-cell therapy into the forefront of immuno-oncology and beyond. The approval of chimeric antigen receptor T-cell modalities has validated the concept that engineered lymphocytes can overcome tumor immune evasion mechanisms and achieve unprecedented rates of complete remission in refractory hematologic cancers. Concurrently, advances in T-cell receptor (TCR) engineering have expanded the target repertoire to include intracellular antigens, offering new therapeutic strategies for solid tumors and viral infections. Tumor-infiltrating lymphocyte (TIL) therapies have further demonstrated clinical benefit by leveraging the patient’s own immune repertoire against diverse tumor types, underscoring the adaptability of adoptive cell approaches.
This wave of innovation has been paralleled by shifts in clinical trial design, regulatory guidance, and reimbursement pathways that encourage expedited development and patient access. Adaptive study protocols, real-world evidence collection, and outcome-based payment models are converging to streamline the approval and commercialization process. Moreover, novel gene-editing platforms, including CRISPR-based systems, are poised to enhance the specificity and safety of T-cell products, while automated manufacturing solutions are reducing production timelines and costs. These synergistic dynamics illustrate how transformative shifts in technology, policy, and commercial strategy are collectively reshaping the T-cell therapy landscape.
Assessing the Economic and Operational Consequences of 2025 United States Tariff Implementation on T-Cell Therapy Supply Chains and Cost Structures
In 2025, the United States enacted a new tariff framework impacting the importation of critical cell culture reagents, viral vectors, and proprietary bioreactor components used in T-cell therapy manufacturing. While designed to bolster domestic production capabilities and safeguard national biosecurity interests, these measures have imposed material cost pressures and logistical complexities on global supply chains. Organizations reliant on cross-border procurement have had to reevaluate sourcing strategies, invest in localized manufacturing partnerships, or absorb additional expenses that ultimately influence pricing and reimbursement negotiations with payers.
Despite these challenges, the cumulative effect of the tariffs has accelerated the establishment of onshore production facilities and incentivized vertical integration across the value chain. Leading contract development and manufacturing organizations have expanded domestic bioreactor capacity and forged strategic alliances to secure uninterrupted supply of critical raw materials. Parallel investments in advanced analytics for supply chain resilience have enabled real-time visibility into inventory levels and tariff exposure, empowering decision-makers to optimize procurement cycles and mitigate cost volatility. Although short-term operational costs have risen, these adaptations are laying the groundwork for a more robust, self-reliant manufacturing ecosystem that can support the anticipated growth of T-cell therapy applications.
Unlocking Market Nuances through Comprehensive Segmentation Analysis of End User Adoption, Cell Sources, Indications, Therapy Modalities, and Manufacturing Models
The diversity of end users adopting T-cell therapies underscores the multifaceted nature of this market. Hospital-based infusion centers serve as the primary administration settings for oncology applications, leveraging clinical infrastructure and multidisciplinary teams to manage complex dosing regimens and monitor for immune-mediated toxicities. Research institutes continue to play a critical role in pioneering novel constructs and validating emerging indications, often serving as the initial proving grounds for experimental therapies. Meanwhile, specialty clinics focused on autoimmune and infectious diseases are facilitating early patient access to investigational T-cell products, demonstrating the technology’s applicability beyond traditional oncology settings.
Cell sourcing decisions-whether to pursue autologous approaches tailored to each patient or allogeneic off-the-shelf constructs-further influence manufacturing and clinical strategies. Autologous modalities offer high specificity and reduced risk of graft-versus-host complications, but entail individualized production processes and extended turnaround times. Allogeneic platforms promise scalable supply and expedited delivery but require additional engineering to mitigate rejection and ensure persistence. Indication-specific segmentation reveals distinct developmental trajectories: autoimmune diseases such as multiple sclerosis, psoriasis, and rheumatoid arthritis are driving exploratory programs that repurpose T cells for immune modulation; infectious disease initiatives targeting hepatitis B, hepatitis C, and HIV are leveraging T-cell cytotoxicity to clear chronic viral reservoirs; and oncology pipelines span hematologic malignancies-including leukemia, lymphoma, and multiple myeloma-as well as solid tumor interventions in breast cancer, lung cancer, and melanoma.
Therapy type choice, from CAR T cell therapy to TCR T cell therapy and TIL therapy, shapes both the clinical profile and manufacturing complexity of products. CAR constructs deliver potent antigen recognition, whereas TCR platforms enable targeting of intracellular antigens and neoantigens. TIL approaches capitalize on the existing tumor-infiltrating repertoire to mount a polyclonal attack, often necessitating bespoke expansion protocols. Underpinning these therapeutic strategies are manufacturing models that range from fully outsourced contract manufacturing to in-house production, with each approach bearing unique implications for scale, quality control, and cost management.
This comprehensive research report categorizes the T-Cell Therapy market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Indication
- Therapy Type
- Manufacturing Model
- Cell Source
- End User
Dissecting Regional Dynamics Shaping T-Cell Therapy Adoption across the Americas, Europe Middle East & Africa, and Asia-Pacific Healthcare Ecosystems
Regional dynamics play a pivotal role in shaping the trajectory of T-cell therapy adoption. In the Americas, a robust pipeline supported by favorable reimbursement guidelines has propelled rapid clinical uptake, particularly in the United States where regulatory incentives and the emergence of specialized treatment centers drive broad access. Canada’s focus on universal healthcare coverage presents both opportunities and challenges as stakeholders negotiate product listings and real-world evidence studies to secure inclusion in public plans. Across Latin America, nascent cell therapy initiatives are emerging, albeit tempered by infrastructure constraints and evolving regulatory frameworks that influence development timelines.
Europe, the Middle East, and Africa encompass a heterogeneous regulatory environment marked by both centralized and country-specific approval pathways. The European Medicines Agency’s conditional marketing authorizations and priority medicines designations have facilitated earlier market entry, while regional centers of excellence in Germany, the United Kingdom, and France serve as hubs for clinical trials and technology transfer. In the Middle East, strategic investments in biotech clusters are accelerating the establishment of manufacturing and clinical research infrastructure, whereas sub-Saharan Africa remains at a formative stage, with pilot programs exploring the feasibility of cell therapy in resource-constrained settings.
The Asia-Pacific region offers a dynamic growth landscape characterized by sizable patient populations and expedited regulatory frameworks. China has emerged as a powerhouse in both CAR T cell approvals and domestic manufacturing capacity, bolstered by substantial government backing. Japan’s pioneering conditional approval pathway for regenerative therapies has incentivized innovative trial designs, while South Korea and Australia are expanding clinical networks to test TCR and TIL approaches. Across Southeast Asia, collaborative consortiums are addressing challenges in cold-chain logistics and workforce training to lay the groundwork for broader deployment.
This comprehensive research report examines key regions that drive the evolution of the T-Cell Therapy market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Profiling Leading Innovators and Emerging Competitors Driving Strategic Alliances, Pipeline Diversification, and Manufacturing Innovations in the Global T-Cell Therapy Arena
Key stakeholders are adopting diverse strategies to secure leadership positions in the T-cell therapy arena. Established biopharmaceutical companies have leveraged robust pipelines and deep expertise in biologics to advance CAR T cell and TCR platforms through late-stage clinical trials and regulatory submissions. At the same time, nimble biotech innovators are carving out niche opportunities by focusing on next-generation constructs that address tumor heterogeneity, mitigate cytokine release syndrome, or employ gene-editing to enhance persistence.
Contract development and manufacturing organizations have become indispensable partners, expanding their footprints with strategically located facilities that accommodate both allogeneic process workflows and personalized autologous production. These service providers are integrating digital manufacturing platforms to enable real-time process monitoring, ensuring compliance with evolving quality standards and accelerating batch release timelines. Additionally, alliances between cell therapy developers and regional healthcare systems have facilitated the establishment of centers of excellence, where multidisciplinary teams collaborate on clinical protocols and patient management best practices.
Meanwhile, technology licensors specializing in viral vector production, gene-editing tools, and closed-system automation are forging licensing and co-development agreements that strengthen the end-to-end value chain. Through these interconnected partnerships, the ecosystem is coalescing around scalable, cost-effective solutions that can meet the growing demand for personalized immunotherapies and expand the reach of T-cell applications to new therapeutic areas.
This comprehensive research report delivers an in-depth overview of the principal market players in the T-Cell Therapy market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Adaptimmune Therapeutics PLC
- Allogene Therapeutics, Inc.
- Amgen Inc.
- AstraZeneca PLC
- Atara Biotherapeutics, Inc.
- Autolus Therapeutics PLC
- Bluebird Bio, Inc.
- Bristol‑Myers Squibb Company
- Caribou Biosciences, Inc.
- Cartesian Therapeutics, Inc.
- Cellectis S.A.
- CRISPR Therapeutics AG
- Fate Therapeutics, Inc.
- Gilead Sciences, Inc.
- Gracell Biotechnologies Inc.
- Immatics Biotechnologies GmbH
- Immunocore Ltd.
- IN8Bio, Inc.
- Johnson & Johnson Services, Inc.
- Mustang Bio, Inc.
- Novartis AG
- Poseida Therapeutics, Inc.
- Precision BioSciences, Inc.
- Tmunity Therapeutics, Inc.
- Vor Biopharma, Inc.
Formulating Strategic Imperatives for Industry Stakeholders to Optimize Portfolio Development, Enhance Manufacturing Resilience, and Expand Market Access in T-Cell Therapy
Stakeholders seeking to navigate the complexities of the T-cell therapy landscape must pursue strategic imperatives that align scientific innovation with operational excellence. First, diversifying therapeutic portfolios to include multiple modality approaches-such as combining CAR T cell therapy with TCR-engineered constructs-can mitigate clinical risk and address heterogeneous patient populations. Real-time collaboration with regulatory authorities to establish rolling review pathways and adaptive trial designs can accelerate time to market while ensuring comprehensive safety assessments.
Investing in flexible manufacturing infrastructure that supports both autologous and allogeneic models will be critical to balancing cost efficiency with supply chain resilience. Organizations should consider modular facility designs and shared production platforms that can be rapidly reconfigured to accommodate new product candidates or shifts in demand. Parallel to this, leveraging advanced data analytics for predictive maintenance, process optimization, and demand forecasting can enhance throughput and reduce batch failures.
Finally, forging cross-sector partnerships-from technology licensors to healthcare delivery networks-will enable a holistic approach to commercialization. Collaborative frameworks for real-world data collection, patient education initiatives, and value-based contracting models can help demonstrate long-term efficacy and build payer confidence. By proactively aligning strategic, operational, and commercial priorities, industry leaders can ensure they are poised to capitalize on the expanding promise of T-cell therapy.
Detailing a Rigorous Multi-Source Research Framework Combining Primary Expert Engagement, Secondary Data Synthesis, and Qualitative Validation for Robust Market Insights
This analysis is grounded in a rigorous research framework that synthesizes insights from multiple primary and secondary sources. Expert interviews with leading clinicians, regulatory advisors, and operations executives provided qualitative depth on clinical trial design, manufacturing innovations, and commercialization strategies. Concurrently, secondary data was systematically gathered from peer-reviewed journals, regulatory agency publications, and industry conference proceedings to validate findings and chart technological trends.
To ensure analytical robustness, data was triangulated through cross-validation exercises that compared proprietary clinical trial registries with public registries and investigator disclosures. Supply chain dynamics were examined by mapping key raw material flows and tariff implications through trade databases and customs filings. Competitive benchmarking incorporated product pipelines, partnership announcements, and facility expansions to construct a comprehensive view of the ecosystem. Throughout the process, methodological rigor was maintained by adhering to established quality protocols, ensuring reproducibility and transparency of the insights presented.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our T-Cell Therapy market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- T-Cell Therapy Market, by Indication
- T-Cell Therapy Market, by Therapy Type
- T-Cell Therapy Market, by Manufacturing Model
- T-Cell Therapy Market, by Cell Source
- T-Cell Therapy Market, by End User
- T-Cell Therapy Market, by Region
- T-Cell Therapy Market, by Group
- T-Cell Therapy Market, by Country
- United States T-Cell Therapy Market
- China T-Cell Therapy Market
- Competitive Landscape
- List of Figures [Total: 17]
- List of Tables [Total: 1590 ]
Synthesizing Key Insights to Illuminate the Transformational Trajectory and Strategic Imperatives of T-Cell Therapy for Decision-Makers in Biopharmaceutical Innovation
Pulling together the core insights of this executive summary reveals a T-cell therapy landscape defined by relentless innovation, evolving regulatory pathways, and strategic imperatives that demand both agility and collaboration. Advances in CAR T, TCR, and TIL modalities underscore the versatility of adoptive cell approaches, while shifts in tariff policy have catalyzed investments in domestic manufacturing resilience. Segmentation analysis highlights the varying clinical needs and operational requirements across end users, cell sources, and indications, underscoring the importance of tailored strategies.
Regional nuances further illustrate how regulatory frameworks, healthcare infrastructure, and payer dynamics shape market entry and adoption. A deep dive into industry players reveals a network of alliances and competitive strategies that span technology licensing, contract manufacturing, and center-of-excellence partnerships. By integrating these diverse perspectives, decision-makers can develop comprehensive roadmaps that align scientific potential with commercial viability.
Ultimately, the trajectory of T-cell therapy will be determined by the ability of stakeholders to anticipate technological breakthroughs, navigate policy landscapes, and optimize operational models. This synthesis serves as a strategic beacon for executives and investors seeking to harness the transformative power of T-cell therapy to deliver meaningful patient outcomes and sustainable business growth.
Connect with Ketan Rohom to Secure This Comprehensive T-Cell Therapy Market Research Report and Empower Strategic Decision-Making in Your Organization
For organizations seeking to refine their competitive edge and catalyze growth in the rapidly evolving realm of T-cell therapy, direct engagement with senior advisory and sales leadership can unlock tailored solutions and deep market intelligence. Reach out to Associate Director of Sales & Marketing Ketan Rohom to explore how this specialized market research, underpinned by rigorous analysis and expert validation, can inform your strategic roadmap. By partnering on a customized implementation plan, stakeholders can translate insights into actionable initiatives, mitigate risk, and accelerate return on investment in cell therapy endeavors.

- How big is the T-Cell Therapy Market?
- What is the T-Cell Therapy Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




