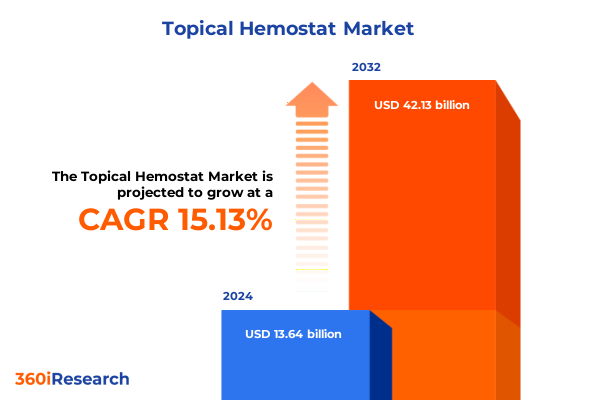
The Topical Hemostat Market size was estimated at USD 15.69 billion in 2025 and expected to reach USD 18.05 billion in 2026, at a CAGR of 15.15% to reach USD 42.13 billion by 2032.
Pioneering the Future of Surgical Bleeding Control with Innovations in Topical Hemostats Driving Clinical and Commercial Transformation
In the rapidly evolving realm of surgical bleeding management, topical hemostats have transitioned from ancillary tools to indispensable solutions for controlling hemorrhage across a spectrum of operative settings. Innovations in biomaterials science and delivery mechanisms are reshaping product portfolios, enabling surgeons to address challenging bleeding scenarios with greater precision and efficiency. As minimally invasive and open procedures alike demand rapid clot formation and tissue compatibility, the role of topical hemostats extends beyond simple hemorrhage control to facilitating improved patient outcomes and streamlined surgical workflows.
Simultaneously, the convergence of clinical evidence, regulatory pathways, and cost-containment imperatives has elevated the strategic importance of topical hemostat products within hospital formularies and ambulatory care protocols. Multidisciplinary teams now evaluate agents not only on hemostatic efficacy but also on ease of use, storage requirements, and integration with electronic health record analytics. This broader perspective drives manufacturers to align research and commercialization efforts with real-world clinical environments, reaffirming the centrality of topical hemostats in modern operative care landscapes.
Emerging Paradigm Shifts in Topical Hemostat Landscape Driven by Technological Breakthroughs and Evolving Clinical Demands
Trauma care advancements and battlefield-driven innovation continue to catalyze new generations of topical hemostatic agents, extending their utility beyond operating rooms into pre-hospital and emergency response contexts. Civilian trauma centers increasingly mirror military protocols, adopting chitosan-impregnated gauzes and advanced sponges that demonstrate rapid clot times under adverse conditions. These applications benefit from tactical guidelines that prioritize rapid deployment, temperature resilience, and shelf stability, thereby widening demand across emergency medical services and public-access bleeding control programs.
Concurrently, regulatory landscapes are tightening evidentiary standards even as accelerated pathways reward products that deliver clear clinical benefits. The FDA’s Safer Technologies Program has facilitated faster clearances for innovative flowable agents, while extended recertification timelines under Europe’s Medical Device Regulation challenge manufacturers to optimize their global launch sequencing. These divergent regulatory dynamics compel organizations to develop agile regulatory strategies, balancing robust clinical data generation with responsive inventory management to mitigate market access delays.
Moreover, digital integration and sustainability considerations are reshaping supply chains and product stewardship. RFID-enabled applicators, real-world performance dashboards, and eco-conscious packaging are emerging as differentiators that align with hospital key performance indicators, bolster value-based procurement, and reinforce long-term clinical trust. As a result, the topology of topical hemostat adoption is shifting from purely efficacy-driven selection toward a multifaceted evaluation encompassing digital analytics, environmental impact, and economic resilience.
Assessing the Cumulative Impact of 2025 U.S. Tariffs on Topical Hemostat Supply Chains, Costs, and Strategic Responses Across the Industry
In 2025, U.S. trade policy measures have imposed stepped tariffs on medical device imports, including consumable hemostat products and critical device components. A 25% duty on certain surgical instruments, respiration equipment, and facemasks has translated into increased procurement costs for hospitals and clinics, while a 20% levy on essential consumables such as syringes and gloves has exerted additional pressure on supply budgets. This shift in duty structures has prompted health systems to reexamine supplier agreements and inventory holdings to absorb initial cost shocks without compromising care delivery.
Further complicating the picture, expanded Section 301 tariffs on Beijing-manufactured medical components have elevated rates up to 100% on items like syringes and needles, disrupting established import channels and undermining China’s competitive position in the U.S. market. As a consequence, U.S. medtech firms face a twofold impact: rising input costs for overseas-produced devices and heightened complexity in global logistics networks. Major suppliers have responded by diversifying supplier bases, stockpiling critical raw materials, and accelerating regional reshoring initiatives to insulate their operations from further tariff escalations.
Given these pressures, leading device manufacturers are optimizing production footprints and reallocating manufacturing capacity to U.S. sites. Firms such as Boston Scientific and Abbott Laboratories have announced significant investments in new domestic facilities to offset anticipated multi-hundred-million-dollar tariff hits in 2025. While immediate cost absorption strategies help maintain margin profiles, long-term resilience depends on cultivating local supply chains, fostering public–private partnerships, and securing tariff exemptions for life-saving devices. In this context, the cumulative effect of U.S. tariffs underscores the strategic imperative for hemostat suppliers to pursue agile supply chain models and proactive trade engagement.
Revealing Comprehensive Segmentation Insights by Product Type, Clinical Application, End User, and Distribution Pathways Shaping Hemostat Market Dynamics
The topical hemostat arena is defined by a rich tapestry of product types, from naturally derived collagen matrices and cyanoacrylate adhesives to gelatin-based hemostatic sponges, oxidized regenerated cellulose in both fabric and powder formats, and potent thrombin formulations. Distinct physicochemical properties influence clotting kinetics, handling characteristics, and compatibility with various tissue surfaces, driving product differentiation strategies.
Clinical application further delineates the market landscape. In cardiovascular surgery, bypass and valve procedures demand flowable agents with rapid adhesion under high-pressure blood flow, while dental specialists rely on extraction and restorative hemostats optimized for confined oral environments. General surgeons select between laparoscopic and open-surgery configurations, seeking products that conform to narrow lumen access or deliver robust hemostasis over larger exposed fields. Adult and pediatric neurosurgeons, as well as orthopedic teams addressing joint replacement and spinal interventions, prioritize formulations with minimal thermal injury profiles and controlled resorption kinetics.
End-user settings add another dimension of complexity. Multi-specialty and orthopedic ambulatory surgery centers require compact, user-friendly kits, whereas dental and dermatology clinics value low-temperature storage requirements and simplified application tools. Private and public hospitals balance formulary breadth with volume discounts, and cardiac or neurology specialty centers mandate stringent traceability and performance data for each applied unit.
Distribution pathways complete the ecosystem. Field and telesales models enable direct engagement with key opinion leaders, while local and national distributors facilitate regional market penetration. Hospital inpatient and outpatient pharmacies manage in-house stock levels, and institutional websites alongside third-party marketplaces offer digital channels for supplemental procurement. Together, these segmentation layers underscore the multifaceted considerations that shape product development and go-to-market strategies.
This comprehensive research report categorizes the Topical Hemostat market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Product Type
- Application
- End User
- Distribution Channel
Unveiling Critical Regional Market Nuances and Growth Drivers Across Americas, Europe Middle East Africa, and Asia-Pacific Hemostatic Solutions
Across the Americas, robust investments in trauma care infrastructure, emergency response programs, and advanced surgical facilities have cemented North America’s leadership in topical hemostat adoption. Public health initiatives and defense-origin protocols permeate civilian settings, ensuring rapid integration of battlefield innovations into mainstream care pathways. Meanwhile, Latin American systems are focused on cost-efficient formulations and multi-community access programs that address both urban and rural surgical needs.
In the Europe, Middle East & Africa region, regulatory harmonization efforts under the European Union Medical Device Regulation coexist with lingering recertification backlogs, influencing launch timing and adoption rates. Regional variation in reimbursement frameworks and hospital procurement practices further drives manufacturers to tailor evidence packages for diverse health-economic environments, from Germany’s bundled DRG models to Gulf Cooperation Council institutions seeking versatile product suites for emerging specialty centers.
Asia-Pacific presents a heterogeneous blend of mature markets and rapidly expanding healthcare ecosystems. Japan and Australia emphasize regenerative composites and precision hemostat applications aligned with aging populations and complex surgical reimbursement structures. Conversely, India, China, and Southeast Asian nations prioritize scalable manufacturing, cost containment, and versatile packaging suited for high-volume general and orthopedic procedures. Strategic partnerships with local distributors and government training initiatives remain central to market access in these burgeoning territories.
This comprehensive research report examines key regions that drive the evolution of the Topical Hemostat market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Evaluating Strategic Movements and Collaborative Innovations of Leading Topical Hemostat Companies in a Competitive Global Arena
Leading global medtech firms are reinforcing their hemostat portfolios through strategic R&D alliances, intellectual property acquisitions, and targeted M&A transactions. Baxter’s dedicated hemostasis laboratories have expanded patent filings around shear-thinning gelatin hybrids, while BD’s procurement of innovative sealant patches broadens its entry into minimally invasive procedural spaces. Cross-licensing agreements between multinational device groups and specialty biopolymer providers are facilitating hybrid formulations that combine scale with niche performance benefits.
Simultaneously, a cohort of agile startups is challenging incumbents with novel biomaterials and smart applicators. Venture-backed firms have secured substantial equity inflows to accelerate trials of temperature-adaptive nanofiber sprays and peptide-crosslinked hydrogels, leveraging cloud-connected registries to validate rapid clot-time performance in diverse clinical settings. These disruptors emphasize data-driven clinical partnerships, enabling swift iteration and real-world feedback that incumbents, constrained by legacy quality systems, find difficult to replicate.
Major medtech players are also deploying dual-sourcing and geographic rebalancing of production to circumvent trade barriers and enhance supply reliability. Investments in new U.S. manufacturing sites by Boston Scientific, Abbott Laboratories, and Siemens Healthineers demonstrate a collective push to anchor critical device production domestically. At the same time, collaborative consortia of academic centers, regulatory bodies, and industry stakeholders are shaping consensus standards for hemostat evaluation, fostering an environment where consistent performance metrics and digital outcome tracking become prerequisites for formulary acceptance.
This comprehensive research report delivers an in-depth overview of the principal market players in the Topical Hemostat market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- B. Braun Melsungen AG
- Baxter International Inc.
- CryoLife, Inc.
- Dilon Technologies Inc
- Integra LifeSciences Corporation
- Johnson & Johnson Services, Inc.
- Medline Industries, LP
- Medtronic plc
- Pfizer Inc.
- Takeda Pharmaceutical Company Limited
- Teleflex Incorporated
Actionable Recommendations for Industry Leaders to Enhance Resilience, Innovate Product Portfolios, and Bolster Market Position in Topical Hemostats
Industry leaders should prioritize the development of integrated supply chain strategies that blend local manufacturing capabilities with diversified global sourcing to mitigate tariff exposure and geopolitical risks. By establishing collaborative procurement frameworks and leveraging multilateral trade agreements, organizations can secure critical raw materials while maintaining cost stability.
Moreover, directing R&D investments toward advanced biomaterials and digital applicator platforms will enable differentiation in increasingly crowded markets. Embedding real-time usage analytics and patient outcome data into product offerings not only reinforces clinical value propositions but also aligns with the shift toward evidence-based purchasing decisions among hospital systems.
Finally, forging strategic alliances with academic research institutions, professional societies, and health-economics experts will expedite regulatory pathways and bolster market access. By co-creating clinical registries and outcome studies, companies can substantiate cost-effectiveness claims and drive sustained adoption across acute care, outpatient, and specialty surgery environments.
Detailing a Rigorous Research Methodology Integrating Primary and Secondary Sources, Expert Validation, and Data Triangulation for Robust Insights
This report synthesizes insights from a rigorous research framework that integrates primary engagements with leading surgeons, procurement specialists, and supply chain managers, alongside a comprehensive review of peer-reviewed literature, regulatory filings, and industry databases. Data triangulation techniques were applied to reconcile quantitative findings from proprietary surveys with qualitative perspectives obtained through expert interviews, ensuring robust validation of market trends and product performance claims.
Additionally, secondary research encompassed an extensive analysis of clinical trial registries, trade association publications, and government policy statements to capture emerging regulatory and reimbursement developments. Proprietary models employed scenario planning to assess the impact of evolving trade policies and technological innovations on market dynamics, while sensitivity analyses confirmed the resilience of key strategic insights under variable conditions.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Topical Hemostat market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Topical Hemostat Market, by Product Type
- Topical Hemostat Market, by Application
- Topical Hemostat Market, by End User
- Topical Hemostat Market, by Distribution Channel
- Topical Hemostat Market, by Region
- Topical Hemostat Market, by Group
- Topical Hemostat Market, by Country
- United States Topical Hemostat Market
- China Topical Hemostat Market
- Competitive Landscape
- List of Figures [Total: 16]
- List of Tables [Total: 3021 ]
Concluding Perspectives on the Topical Hemostat Market Highlighting Key Insights, Challenges, and the Path Forward for Stakeholders
The topical hemostat market stands at the intersection of technological innovation, shifting clinical protocols, and evolving trade landscapes. Key product segments continue to diversify, meeting the nuanced demands of surgical specialties and care settings, while regulatory harmonization and digital integration reshape access pathways.
Stakeholders face dual imperatives: sustaining innovation pipelines that deliver clear clinical benefits and fortifying supply chains to withstand policy-driven disruptions. As market competition intensifies, success will favor organizations that couple agile operational models with deep clinical partnerships and data-centric product propositions. Going forward, the capacity to anticipate regulatory shifts, harness advanced biomaterials, and embed real-world analytics will define leadership in the topical hemostat domain.
Take the Next Step Today Reach Out to Ketan Rohom Associate Director Sales Marketing to Acquire Comprehensive Topical Hemostat Market Intelligence
The insights presented throughout this executive summary underscore the dynamic shifts, segmentation nuances, regulatory challenges, and competitive maneuvers that characterize the topical hemostat arena. As the industry continues to adapt to evolving clinical demands, policy developments, and supply chain pressures, stakeholders must align their strategies with resilient operational models, data-driven innovation, and targeted market engagement. To gain a deeper understanding of the comprehensive analysis, detailed findings, and proprietary data that inform these conclusions, we invite you to connect with Ketan Rohom, Associate Director, Sales & Marketing, to secure access to the full market research report and empower your organization with actionable intelligence for success in the topical hemostat market.

- How big is the Topical Hemostat Market?
- What is the Topical Hemostat Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




