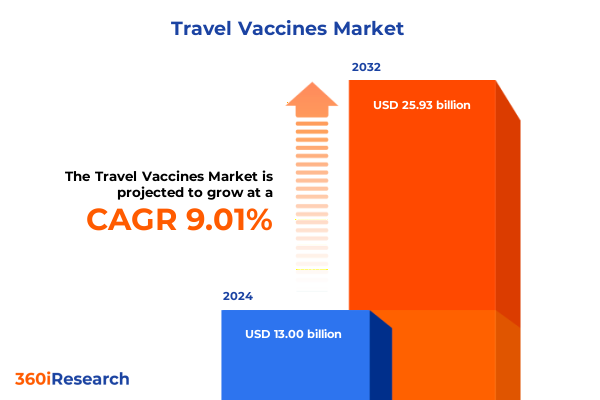
The Travel Vaccines Market size was estimated at USD 14.13 billion in 2025 and expected to reach USD 15.36 billion in 2026, at a CAGR of 9.06% to reach USD 25.93 billion by 2032.
Laying the Critical Groundwork for a Resilient and Responsive Global Travel Vaccine Ecosystem in an Era of Unprecedented Mobility
Travelers today navigate an increasingly complex global landscape, where the risks of infectious diseases intersect with the growing desire for exploration and cultural exchange. As passenger volumes rebound and international corridors reopen fully, the role of immunization has never been more critical. Vaccine developers, distributors, and public health stakeholders must collectively respond to a surge in demand for targeted preventive solutions that ensure safe passage across borders.
In this context, precision in vaccine selection, efficacy against emerging pathogens, and accessibility of administration channels define success. The rise of technologically advanced formulations and digital health tools is reshaping expectations around immunization. Meanwhile, travelers are more informed and discerning, seeking assurance through proven safety profiles and streamlined vaccination processes. This introduction sets the stage by contextualizing the multifaceted challenges and opportunities within the global travel vaccine arena, underscoring the imperative of agile strategies that align with shifting traveler protections and public health objectives.
Uncovering the Transformative Forces Redefining Travel Vaccination Strategies and Technologies in a Post-Pandemic World of Traveler Diversity
In recent years, a constellation of shifts has converged to redefine how travel vaccines are developed, distributed, and administered. The aftermath of widespread viral outbreaks accelerated innovation in messenger RNA platforms, demonstrating promise beyond pandemic-specific applications and opening doors for future vaccine candidates. Concurrently, the integration of digital health records and mobile immunization certificates is streamlining verification processes, enabling seamless traveler experiences at checkpoints and reducing administrative burdens for healthcare providers.
Pharmaceutical companies are forging alliances with technology firms to harness data analytics for predictive modeling, targeting vaccination drives toward high-risk routes and populations. At the same time, consumer behavior has evolved; travelers now seek holistic wellness experiences that encompass personalized immunization plans, telehealth consultations, and post-vaccination monitoring services. As a result, traditional vaccination channels are pivoting toward hybrid models that blend in-person care with virtual engagement, ensuring continuity of follow-up and reinforcing adherence to recommended schedules.
These transformative forces collectively underscore a departure from one-size-fits-all vaccine programs. Instead, the landscape is moving rapidly toward tailored, data-driven strategies that prioritize both efficacy and convenience, positioning the travel vaccine ecosystem for sustained growth and resilience.
Assessing the Far-Reaching Consequences of United States Tariff Adjustments on Vaccine Supply Chains and Industry Competitiveness through 2025
In 2025, adjustments to import tariffs imposed by federal authorities have exerted a notable influence on the travel vaccine supply chain and the economics of production. Increased duties on active pharmaceutical ingredients have elevated manufacturing costs for certain biologics, prompting companies to reassess their sourcing strategies and consider reshoring critical production processes. This shift has led to a recalibration of global procurement networks, with some manufacturers forging new partnerships with domestic suppliers to mitigate exposure to foreign tariff fluctuations.
Meanwhile, import levies on ancillary materials such as vials, syringes, and specialized packaging have driven demand for locally produced alternatives. While this trend has stimulated investment in domestic manufacturing capacity, it has also placed pressure on smaller contract manufacturers to upgrade facilities in order to comply with stringent quality and regulatory standards. In response, several global immunology leaders have scaled up their footprint within the United States, bolstering production redundancy and shortening lead times for priority vaccines.
Taken together, these tariff dynamics are reshaping competitive positioning across the value chain. Stakeholders are confronted with a strategic imperative: adapt supply networks for cost effectiveness and resilience, while ensuring that immunization programs remain accessible and sustainable in the face of evolving trade policies.
Extracting Actionable Perspectives from Comprehensive Market Segmentation That Illuminate Vaccine Preferences by Type Form Age Group and Distribution Channels
A nuanced examination of the travel vaccine market reveals divergent behaviors and preferences when analyzed by vaccine type, form, age group, and distribution channel. Hepatitis A and B immunizations continue to represent foundational offerings, particularly among travelers to regions where sanitation challenges persist. Influenza vaccines are increasingly sought by those on seasonal tours, while meningococcal and rabies prophylactics register heightened interest among adventure seekers and medical mission participants. Typhoid and yellow fever vaccines retain critical importance for travelers to endemic zones, reflecting the enduring burden of vector-borne and waterborne illnesses.
Delivery preferences exhibit clear bifurcation between injectable formats and emerging oral formulations. Injectable vaccines maintain their dominance in clinical settings, driven by established efficacy data and ongoing provider familiarity. However, oral options are gaining traction for their convenience, particularly among mobile populations that prioritize ease of self-administration and reduced clinic visits. Age segmentation further accentuates market nuances; adult travelers favor combination and multi-pathogen shots, whereas pediatric immunization protocols lean toward age-specific dosage formulations and specialized administration guidelines.
Across end users, hospitals and clinics remain pivotal for in-depth patient evaluation and complex vaccine regimens. Pharmacies and travel clinics have become indispensable for last-minute immunization needs and comprehensive pre-travel consultations. Meanwhile, online pharmacies are carving out a niche by offering direct-to-consumer access and digital reminders, underscoring the interplay between e-commerce platforms and traditional healthcare providers in fulfilling traveler vaccine requirements.
This comprehensive research report categorizes the Travel Vaccines market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Vaccine Type
- Form
- Age Group
- End User
Delineating Critical Regional Variations Illuminating Demand Drivers and Stakeholder Dynamics across the Americas EMEA and Asia-Pacific
Regional dynamics in the travel vaccine landscape reflect varying public health frameworks, disease prevalence, and traveler demographics across the Americas, the combined Europe Middle East and Africa region, and Asia-Pacific. In the Americas, proactive immunization policies and robust insurance coverage programs contribute to high uptake of both standard and novel vaccine offerings. The mature healthcare infrastructure supports rapid deployment of updated formulations, while widespread digital health adoption streamlines appointment scheduling and post-vaccination monitoring.
In Europe Middle East and Africa, a mosaic of regulatory environments influences vaccine accessibility. Western European nations emphasize strict pharmacovigilance and data privacy, leading to comprehensive safety reporting mechanisms. Meanwhile, several Middle Eastern countries are emerging as distribution hubs, leveraging strategic geographic positioning to serve both inbound pilgrims and outbound tourists. Across Sub-Saharan Africa, logistical hurdles persist for remote communities, heightening the need for mobile clinics and targeted outreach campaigns to ensure travelers receive critical protection.
Asia-Pacific presents a dynamic interplay of high-volume vaccine production and rapidly evolving consumer expectations. Leading economies in the region are scaling local manufacturing capacity to meet domestic demand and export opportunities. Concurrently, elevated awareness around public health readiness is driving collaboration between governments and private stakeholders to enhance cold chain networks and telemedicine capabilities. As a result, the region stands out for its agile response to emergent health threats and commitment to integrating cutting-edge technologies into travel immunization protocols.
This comprehensive research report examines key regions that drive the evolution of the Travel Vaccines market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Analyzing Strategic Moves and Competitive Positioning of Leading Biopharmaceutical Innovators Shaping the Future of Travel Immunization
Leading biopharmaceutical companies are deploying diversified strategies to maintain competitive advantage and extend their foothold in the travel vaccine market. Major global players with extensive R&D pipelines are emphasizing next-generation platforms, including recombinant protein and mRNA constructs, to broaden protective breadth and accelerate regulatory approval timelines. Partnerships with academic institutions and biotech startups are fostering breakthrough discoveries, while licensing agreements are expediting market entry for promising candidates in late-stage development.
Concurrently, several pharmaceutical giants are optimizing distribution networks through strategic collaborations with logistics specialists and regional health agencies. By integrating end-to-end cold chain traceability and real-time inventory management, they are enhancing supply predictability and reducing vaccine wastage. Investments in digital engagement tools, such as AI-driven demand forecasting and virtual pre-travel consultations, are reinforcing customer loyalty and strengthening provider relationships.
In parallel, niche innovators are capitalizing on specialized formulations and targeted administration devices to serve distinct traveler cohorts. Whether focusing on high-efficacy adjuvanted vaccines or compact oral delivery systems, these agile entities are carving out market niches that complement the portfolios of larger incumbents. This competitive mosaic underscores the importance of both scale and specialization in shaping how travel immunization offerings evolve.
This comprehensive research report delivers an in-depth overview of the principal market players in the Travel Vaccines market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Abbott Laboratories
- AstraZeneca PLC
- Bavarian Nordic A/S
- Bharat Biotech International Limited
- Bio-Manguinhos
- Bio-Med (P) Limited
- Biological E Limited
- Cadila Pharmaceuticals Limited
- CSL Limited
- Dano Vaccines & Biologicals Private Limited
- Dynavax Technologies Corporation
- Emergent BioSolutions Inc.
- GlaxoSmithKline plc
- Incepta Pharmaceuticals Ltd.
- Indian Immunologicals Ltd.
- Johnson & Johnson Services, Inc
- Merck & Co., Inc.
- Novartis AG
- Perusahaan Umum (Persero) Bio Farma
- Pfizer Inc.
- Sanofi S.A.
- Serum Institute of India Pvt. Ltd.
- Shenzhen Kangtai Biological Products Co., Ltd.
- Takeda Pharmaceutical Company Limited
- Valneva SE
- Walvax Biotechnology Co., Ltd.
Delivering Actionable Strategic Imperatives Guiding Stakeholders to Unlock New Opportunities and Strengthen Market Resilience in Vaccination
Industry leaders can elevate their competitive standing and drive sustainable growth by embracing three interconnected strategic imperatives. First, enhance digital engagement by deploying integrated platforms that offer seamless pre-travel assessments, automated vaccine reminders, and telehealth follow-up services. This approach not only increases adherence to vaccination schedules but also fosters ongoing customer engagement and satisfaction.
Second, diversify product portfolios by advancing oral and combination vaccines that meet the needs of time-sensitive travelers and those seeking minimal clinic interactions. Streamlining regulatory pathways for innovative delivery methods will be crucial, as will forging partnerships with distribution networks that can rapidly mobilize new offerings in response to changing epidemiological trends.
Finally, strengthen supply chain resilience through regional manufacturing alliances and flexible procurement frameworks. By establishing fallback options for manufacturing and leveraging advanced analytics for demand planning, organizations can mitigate the impact of trade policy shifts or logistical disruptions. Collectively, these actionable recommendations will enable stakeholders to adapt swiftly to market forces and capitalize on emerging opportunities in the travel vaccine sector.
Revealing Rigorous Methodological Approaches Employed to Ensure Comprehensive Insights and Data Integrity in Travel Vaccine Research
Our research framework is built upon a rigorous blend of primary and secondary data collection, ensuring that insights are both comprehensive and credible. Primary interviews with key opinion leaders, including immunologists, global health officials, and distribution executives, provided firsthand perspectives on market trends, regulatory developments, and operational challenges. These qualitative inputs were complemented by quantitative surveys of travelers, healthcare providers, and procurement specialists, yielding a robust data set for analysis.
Secondary research involved an exhaustive review of peer-reviewed journals, regulatory filings, conference proceedings, and publicly accessible health databases to corroborate emerging trends and validate market behaviors. Cross-validation techniques were applied to reconcile discrepancies between data sources, ensuring consistency across geographic regions and across different end-user segments.
Analytical rigor was further enhanced through scenario modeling and sensitivity analyses, which evaluated the potential impact of variables such as policy changes, supply chain disruptions, and technological breakthroughs. The fusion of methodological transparency and data triangulation underpins the reliability of our findings, empowering stakeholders to make informed strategic decisions.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Travel Vaccines market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Travel Vaccines Market, by Vaccine Type
- Travel Vaccines Market, by Form
- Travel Vaccines Market, by Age Group
- Travel Vaccines Market, by End User
- Travel Vaccines Market, by Region
- Travel Vaccines Market, by Group
- Travel Vaccines Market, by Country
- United States Travel Vaccines Market
- China Travel Vaccines Market
- Competitive Landscape
- List of Figures [Total: 16]
- List of Tables [Total: 795 ]
Concluding Insights Emphasizing Strategic Adaptability Insights and Collaborative Pathways to Navigate Future Challenges in Travel Vaccination
As the global travel vaccine ecosystem continues to evolve, strategic adaptability remains the cornerstone of long-term success. Stakeholders must remain vigilant in monitoring geopolitical developments, public health advisories, and technological innovations that could reshape market dynamics. By fostering collaborative networks across industry, government, and healthcare providers, organizations can expedite the translation of scientific advances into practical immunization solutions.
Maintaining agility in product development and distribution strategies will be essential to navigate potential tariff fluctuations, supply chain complexities, and shifting traveler expectations. Moreover, a commitment to data-driven decision-making and continuous stakeholder engagement will enable a proactive response to emergent threats, ensuring that traveler health remains protected and global mobility can be sustained.
Ultimately, the ability to balance innovation with operational resilience, while delivering high-quality immunization experiences, will determine which players lead the next phase of growth. Equipped with the insights and recommendations presented here, industry participants are well-positioned to chart a course through uncertainty and capitalize on evolving demand patterns.
Seizing Opportunities and Empowering Leaders to Access Deep-Dive Travel Vaccine Market Insights through Engagement with Associate Director Sales and Marketing
Engaging directly with Ketan Rohom, Associate Director Sales and Marketing, is the most effective way to unlock the full benefits of our in-depth travel vaccine market report. By collaborating with Mr. Rohom, you will gain personalized guidance on how to interpret our comprehensive findings and tailor strategies to your organization’s unique objectives. His expertise will help you navigate complex insights, refine key initiatives, and identify high-impact growth avenues to outpace competitors.
Connect with Ketan Rohom to secure your access to robust market data and executive-level analysis that address regulatory nuances, evolving traveler behaviors, and supply chain intricacies. Through this engagement, you will receive a bespoke overview of the report’s core strengths, ensuring you extract maximum value from every section. Taking this step will empower your leadership team with the intelligence needed to drive sustainable growth and innovation in the dynamic travel vaccine market.

- How big is the Travel Vaccines Market?
- What is the Travel Vaccines Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




