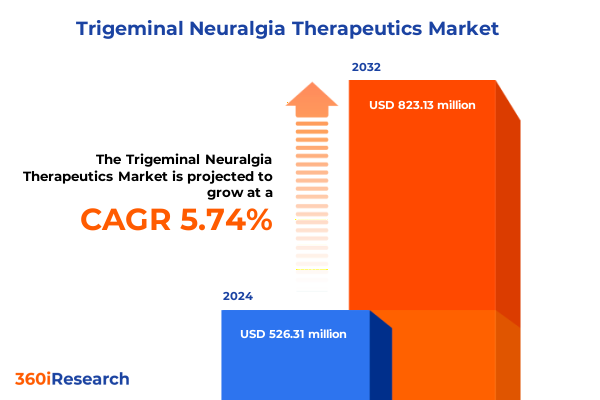
The Trigeminal Neuralgia Therapeutics Market size was estimated at USD 555.73 million in 2025 and expected to reach USD 587.02 million in 2026, at a CAGR of 5.77% to reach USD 823.13 million by 2032.
Pioneering insights into trigeminal neuralgia therapeutics unveiling the foundation for breakthrough patient-centered care approaches
Trigeminal neuralgia remains one of the most debilitating facial pain disorders characterized by sudden recurrent episodes of intense discomfort that significantly diminish patient well-being and quality of life. This executive summary delivers a strategic overview of the evolving therapeutic landscape by exploring how clinical advancements and shifting stakeholder priorities are converging to reshape care pathways. Recent progress has emerged across pharmacologic treatments surgical interventions and complementary modalities emphasizing the imperative for more effective long-lasting relief with fewer adverse effects. By situating these developments within a broader context of healthcare innovation this introduction establishes the framework for a deep-dive analysis into market dynamics regulatory influences and competitive strategies.
In navigating the complexity of trigeminal neuralgia management decision-makers must consider multidisciplinary approaches that integrate established anticonvulsants with novel techniques such as peripheral nerve stimulation and targeted surgical procedures. Furthermore the advent of personalized care models underscores the importance of patient segmentation and tailored therapeutic regimens. To guide stakeholders through this multifaceted environment the report systematically examines tariff implications segmentation insights regional trends company strategies and actionable recommendations. The resulting narrative equips industry executives healthcare professionals and policy planners with a robust foundation for informed decision-making and strategic planning.
Designed to cater to experts and innovators alike this introduction sets the stage for a comprehensive assessment of the market forces driving the next generation of trigeminal neuralgia therapies. It underscores the report’s commitment to delivering rigorous objective and forward-thinking intelligence that addresses both clinical imperatives and commercial opportunities
Revolutionary technological and clinical shifts reshaping the trigeminal neuralgia management landscape with precision targeted innovations
The therapeutics landscape for trigeminal neuralgia is undergoing a profound transformation driven by technological breakthroughs novel pharmacologic targets and a shift toward minimally invasive procedures. Innovations in neuromodulation have challenged traditional paradigms by offering precision targeting of nerve pathways to disrupt pain signaling without extensive tissue damage. Concurrently the refinement of stereotactic radiosurgery and advances in imaging guidance have elevated the safety and efficacy of surgical interventions. Beyond procedural progress the emergence of combination regimens that synergize anticonvulsants with complementary therapies reflects an integrative philosophy aimed at holistic patient care.
Complementing these clinical innovations are data-driven decision-support tools that leverage real-world evidence and predictive analytics to optimize treatment selection. The integration of digital health platforms and remote monitoring applications is enhancing patient engagement and adherence while generating valuable insights into long-term outcomes. These technological and clinical shifts are further catalyzed by evolving reimbursement frameworks that increasingly recognize the value of durable pain relief and quality-of-life improvements. As a result decision-makers must reconcile rapid innovation cycles with regulatory and payer expectations to successfully navigate the commercialization pathway.
Through this exploration of transformative shifts the report illuminates how agility in clinical development and strategic collaboration across sectors are becoming critical success factors. Stakeholders equipped with a clear understanding of these emerging dynamics will be best positioned to capitalize on the next wave of therapeutic breakthroughs and deliver meaningful improvements in patient outcomes
Examining the extensive effects of newly implemented United States tariffs on supply chain stability and pricing dynamics in 2025
The implementation of new United States tariffs in early 2025 has introduced a complex layer of trade policy considerations for manufacturers and distributors within the trigeminal neuralgia therapeutics market. Tariff adjustments impacting imported pharmaceutical ingredients and medical device components have increased production costs and created pressure on supply chain efficiency. Companies reliant on overseas sourcing of active pharmaceutical ingredients have faced the dual challenge of securing alternative suppliers and negotiating with raw material providers to mitigate price escalation. In turn these strategic responses underscore the critical need for a resilient procurement framework and robust supplier diversification initiatives.
Beyond raw material procurement the tariffs have reverberated across manufacturing operations prompting stakeholders to reassess domestic production capacities and potential reshoring strategies. Facility investments and localized assembly processes are being evaluated to preserve product affordability while maintaining adherence to stringent quality standards. Moreover the shifting cost structure has accelerated partnerships between industry players and contract manufacturing organizations that can offer scalable domestic capabilities. Payers and healthcare providers are also adapting, with formulary committees increasingly scrutinizing cost-effectiveness and total treatment expenditure, reinforcing the linkage between pricing policy and patient access.
Looking ahead stakeholders must continuously monitor tariff policy developments and engage proactively with trade associations and regulatory bodies. This vigilance will enable timely adjustments to sourcing strategies pricing models and risk management protocols, ensuring sustained supply continuity and the capacity to respond adeptly to future policy shifts affecting the trigeminal neuralgia therapeutics sector
Deep dive into comprehensive segmentation revealing critical insights across therapy modalities product types mechanisms distribution channels and end users
The market for trigeminal neuralgia therapeutics is stratified across multiple analytical dimensions each offering unique insights into treatment adoption and competitive positioning. Based on therapy type the landscape encompasses complementary therapies neuromodulation pharmacologic interventions and surgical procedures. Within complementary therapies modalities such as acupuncture herbal medicine and physical therapy are gaining traction among patients seeking holistic pain management alongside conventional care. The neuromodulation segment is advancing rapidly driven by deep brain stimulation and peripheral nerve stimulation technologies designed to modulate aberrant neural activity with precision. Pharmacologic treatments remain foundational with established classes of analgesics anticonvulsants and muscle relaxants. Within anticonvulsants agents like carbamazepine gabapentin lamotrigine and oxcarbazepine continue to lead prescribing patterns based on tolerability and efficacy profiles. In parallel surgical approaches such as balloon compression gamma knife radiosurgery microvascular decompression and radiofrequency ablation persist as vital options for refractory cases.
Examining product type reveals a dual focus on branded and generic formulations. Branded therapeutics driven by innovator and patented entities emphasize proprietary delivery mechanisms and extended-release profiles that differentiate value propositions in a crowded landscape. Mechanism-of-action segmentation further illuminates preference trends among calcium channel blockers gaba analogues nmda receptor antagonists and voltage-gated sodium channel blockers, each offering distinct neural modulation pathways. Distribution channels span hospital pharmacies online pharmacies and retail pharmacies reflecting evolving patient acquisition preferences and the rising prominence of e-commerce in medication fulfillment. Finally end user segmentation highlights treatment delivery environments ranging from ambulatory surgical centers and home care settings to hospitals and specialty clinics where dedicated expertise informs procedural selection and post-operative management approaches
This comprehensive research report categorizes the Trigeminal Neuralgia Therapeutics market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Therapy Type
- Route Of Administration
- Mechanism Of Action
- Patient Age Group
- Distribution Channel
- End User
Granular regional perspectives highlighting diverse trends opportunities and challenges across the Americas Europe Middle East Africa and Asia Pacific
Regional dynamics in the trigeminal neuralgia therapeutics market reveal distinct patterns of growth regulatory frameworks and patient access considerations across the Americas Europe Middle East & Africa and Asia-Pacific regions. In the Americas advanced healthcare infrastructure coupled with favorable reimbursement environments has supported robust adoption of neuromodulation technologies and innovative pharmacologic formulations. Additionally progressive clinical guidelines and strong collaborative networks among academic centers and industry sponsors are accelerating the pace of clinical trials and real-world evidence generation.
In the Europe Middle East & Africa region diverse regulatory requirements and variable market access pathways necessitate tailored commercialization strategies. Countries with centralized reimbursement agencies demonstrate streamlined adoption of high-value therapies while emerging markets within the region present significant expansion opportunities driven by increasing awareness and improving healthcare expenditure. Stakeholders operating in this region must adeptly navigate localized pricing negotiations and engage with patient advocacy groups to build durable market presence.
The Asia-Pacific region is characterized by high patient volumes rising healthcare investment and an expanding network of specialized clinics. Governments across key markets are incentivizing research into neurological disorders and providing grants to support domestic production of essential drug components. At the same time logistical considerations such as import regulations and distribution network optimization remain critical to ensuring timely access for end users. By aligning product portfolios and strategic alliances with regional healthcare priorities stakeholders can effectively leverage the growth trajectory of the trigeminal neuralgia therapeutics landscape in this dynamic region
This comprehensive research report examines key regions that drive the evolution of the Trigeminal Neuralgia Therapeutics market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Profiling leading players and emerging innovators driving collaborations pipeline expansion and competitive dynamics in therapeutic solutions
Leading pharmaceutical and medical device companies are actively shaping the competitive dynamics of the trigeminal neuralgia therapeutics market through strategic partnerships pipeline expansion and technology acquisitions. Established industry players continue to invest in life-cycle management of key pharmacologic assets while pursuing label extensions to address refractory neuralgia subtypes. Concurrently device manufacturers are enhancing their portfolios by integrating digital connectivity features and AI-driven treatment planning tools to differentiate neuromodulation platforms and surgical systems.
Emerging innovators are gaining traction by targeting niche segments within the disease spectrum through precision medicine approaches and biomarker-driven therapeutic development. These agile entrants often collaborate with research institutions to accelerate early-stage studies and leverage grant funding for proof-of-concept trials. In addition contract research organizations and specialized service providers play an increasingly vital role by offering integrated development services that bridge clinical research with regulatory consulting and commercial strategy. Mergers and acquisitions activities underscore the drive for vertical integration, with market leaders seeking to combine complementary capabilities across drug formulation, device engineering and digital health to deliver end-to-end solutions for patients and providers
This comprehensive research report delivers an in-depth overview of the principal market players in the Trigeminal Neuralgia Therapeutics market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Abbott Laboratories
- AbbVie Inc.
- Alembic Pharmaceuticals Limited
- Apotex Inc.
- Biogen Inc.
- Biohaven Pharmaceuticals Holding Company Ltd.
- Boston Scientific Corporation
- Elekta AB
- GlaxoSmithKline plc
- Grünenthal GmbH
- Initiator Pharma AS
- Ipsen
- Kriya Therapeutics, Inc.
- Lupin Limited
- Noema Pharma AG
- Novartis AG
- Pfizer Inc.
- Sun Pharmaceutical Industries Ltd.
- Supernus Pharmaceuticals, Inc.
- Takeda Pharmaceutical Company Limited
- Taro Pharmaceutical Industries Ltd.
- Teva Pharmaceutical Industries Ltd.
- Torrent Pharmaceuticals Ltd.
- UCB S.A.
- Vertex Pharmaceuticals Incorporated
Strategic actionable recommendations empowering industry leaders to navigate complex market dynamics and maximize therapeutic value
Industry leaders must adopt a multi-pronged strategy to capitalize on evolving opportunities and mitigate emerging risks in the trigeminal neuralgia therapeutics market. First, fostering strategic alliances with academic research centers and technology startups will enable accelerated access to cutting-edge modalities while sharing the financial and operational risks of early-stage development. Second, investing in flexible manufacturing capabilities and supplier diversification will safeguard supply chain resilience in the wake of shifting trade policies and raw material constraints. Third, prioritizing real-world evidence generation through patient registries and post-marketing surveillance initiatives will strengthen reimbursement positioning and reinforce the value narrative among payers.
Additionally companies should leverage digital health platforms to enhance patient engagement and adherence by integrating telemedicine follow-up and remote monitoring into care pathways. Tailored market access strategies that reflect regional regulatory nuances and payer requirements will be critical to unlocking growth across diverse geographies. Finally embedding environmental social governance principles into corporate strategy can differentiate brands in a competitive environment increasingly driven by stakeholder scrutiny of ethical and sustainability commitments. By executing these actionable recommendations decision-makers will be well-positioned to drive innovation deliver superior patient outcomes and achieve sustainable commercial success
Robust methodological framework detailing multisource data synthesis analytical techniques and validation processes ensuring research rigor
The research methodology underpinning this analysis integrates multiple data streams to ensure robustness validity and comprehensive market coverage. Primary research included in-depth interviews with key opinion leaders clinical practitioners and senior executives from pharma and device companies to capture firsthand perspectives on therapy adoption challenges and emerging trends. Secondary research sources encompassed peer-reviewed journals clinical trial registries regulatory filings and trade publications to validate technology developments and competitive intelligence.
Analytical techniques applied to the aggregated data involved both qualitative thematic analysis and quantitative triangulation. Thematic analysis facilitated identification of strategic imperatives and market drivers while quantitative triangulation cross-verified insights against historical benchmarks and real-world utilization data. Geospatial mapping of tariff impacts and supply chain disruptions enabled visualization of regional vulnerabilities. Rigorous validation processes included stakeholder workshops and expert panel reviews to refine findings and ensure alignment with industry consensus. The resulting methodological framework delivers a transparent reproducible and defensible foundation for strategic decision-making within the trigeminal neuralgia therapeutics space
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Trigeminal Neuralgia Therapeutics market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Trigeminal Neuralgia Therapeutics Market, by Therapy Type
- Trigeminal Neuralgia Therapeutics Market, by Route Of Administration
- Trigeminal Neuralgia Therapeutics Market, by Mechanism Of Action
- Trigeminal Neuralgia Therapeutics Market, by Patient Age Group
- Trigeminal Neuralgia Therapeutics Market, by Distribution Channel
- Trigeminal Neuralgia Therapeutics Market, by End User
- Trigeminal Neuralgia Therapeutics Market, by Region
- Trigeminal Neuralgia Therapeutics Market, by Group
- Trigeminal Neuralgia Therapeutics Market, by Country
- United States Trigeminal Neuralgia Therapeutics Market
- China Trigeminal Neuralgia Therapeutics Market
- Competitive Landscape
- List of Figures [Total: 18]
- List of Tables [Total: 2067 ]
Synthesizing key findings and implications to solidify future directions and strategic imperatives in trigeminal neuralgia therapeutics
This executive summary has elucidated the key market dynamics shaping the future of trigeminal neuralgia therapeutics spanning transformative technological shifts tariff-driven supply chain considerations nuanced segmentation insights regional variability and competitive landscapes. Stakeholders are now equipped with a holistic understanding of how integrated therapeutic approaches and strategic partnerships can elevate patient outcomes while sustaining commercial viability. The intersection of innovation in neuromodulation surgical precision and pharmacologic refinements sets the stage for unprecedented progress in pain management and quality of life enhancements.
As decision-makers chart their strategic course, maintaining agility in response to regulatory adjustments and trade policy developments will be essential. Leveraging the analytical rigor and forward-looking perspectives presented here organizations can anticipate emerging opportunities and mitigate potential disruptions. The insights distilled offer a clear pathway for aligning R&D efforts with market needs optimizing commercial strategies and fostering collaborations that accelerate the delivery of next-generation therapies. In synthesis this summary underscores the promise of a patient-centric paradigm that harnesses multidisciplinary expertise to transform the standard of care for individuals living with trigeminal neuralgia
Connect directly with Ketan Rohom to secure the complete Trigeminal Neuralgia Therapeutics report and propel strategic growth initiatives
Unlock unparalleled insights and strategic foresight by connecting with Ketan Rohom Associate Director Sales & Marketing at 360iResearch to acquire the comprehensive Trigeminal Neuralgia Therapeutics market research report Your direct engagement will provide access to proprietary data detailed segmentation analyses and forward-looking strategic recommendations essential for driving competitive advantage and accelerating growth initiatives By partnering with Ketan Rohom you will secure personalized guidance on report customization pricing options and implementation frameworks that align with your organizational objectives Reach out today to transform market intelligence into actionable strategies and position your enterprise at the forefront of therapeutic innovation in trigeminal neuralgia

- How big is the Trigeminal Neuralgia Therapeutics Market?
- What is the Trigeminal Neuralgia Therapeutics Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




