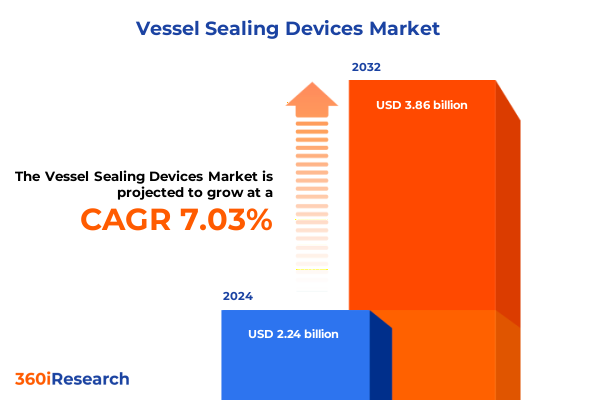
The Vessel Sealing Devices Market size was estimated at USD 2.38 billion in 2025 and expected to reach USD 2.54 billion in 2026, at a CAGR of 7.12% to reach USD 3.86 billion by 2032.
Overview of Vessel Sealing Device Dynamics Shaping Surgical Innovation and Patient Outcomes in the Modern Healthcare Environment
Vessel sealing devices have emerged as indispensable tools in modern surgical procedures, offering clinicians enhanced control over hemostasis and tissue management. These advanced energy-based instruments optimize procedural efficiency by combining thermal and mechanical energy modalities to achieve rapid vessel occlusion with minimal collateral damage. The growing emphasis on patient safety and reduced operative times has fueled clinician preference for these solutions, positioning vessel sealing devices at the forefront of surgical innovation.¹²
In recent years, the rapid expansion of minimally invasive surgeries has underscored the critical role of vessel sealing technologies in reducing intraoperative blood loss and postoperative complications. As hospitals strive to improve patient outcomes and streamline care pathways, the adoption of these devices has been closely linked to higher procedural success rates and shorter hospital stays.³ This trend has prompted device manufacturers to accelerate product development cycles, ensuring their portfolios address evolving clinician and hospital requirements.
Moreover, heightened R&D investments have led to a proliferation of hybrid and multifunctional platforms that integrate bipolar energy, ultrasonic sealing, and advanced feedback systems. With an increasing array of single-use and reusable instrument configurations, hospitals and ambulatory centers can align device selection with procedural volumes and cost control objectives. This expanded range underscores a dynamic landscape where technological differentiation and clinical evidence remain paramount.
Exploring Transformative Innovations and Technological Breakthroughs Revolutionizing Vessel Sealing Practices Across Energy Modalities and Surgical Platforms
The vessel sealing domain is witnessing a paradigm shift driven by converging technological breakthroughs and evolving surgical practices. Hybrid energy platforms that seamlessly blend bipolar and ultrasonic modalities empower surgeons to achieve faster sealing and cutting, significantly speeding up operative workflows. These innovations are complemented by integrated feedback algorithms that dynamically adjust energy delivery to tissue impedance, thereby reducing thermal spread and preserving adjacent structures.⁴
Simultaneously, the integration of vessel sealing instruments into robotic surgical platforms has unlocked new horizons for precision and ergonomics. Enhanced articulation and real-time visual feedback allow for more accurate dissection in confined anatomical regions, particularly in complex cardiovascular and urologic procedures. Such capabilities are fostering synergies between robotics and advanced energy systems, leading to next-generation solutions that emphasize both safety and efficiency.
In parallel, digital health and connectivity features are enabling predictive maintenance, remote performance monitoring, and data-driven insights. Manufacturers are embedding sensors in generators and handpieces to capture usage metrics, facilitating service optimization and enabling hospitals to align device deployment with clinical demand. As a result, the device landscape is transitioning from standalone instruments to interconnected ecosystems that support evidence-based surgery and continuous performance improvement.
Assessing the Broad Implications of United States Trade Measures and Tariff Adjustments on the Vessel Sealing Device Supply Chain and Market Dynamics
In 2025, United States trade policy shifts have introduced a complex array of import duties that impact medical device supply chains, including components used in vessel sealing technologies. Section 301 tariff adjustments on certain Chinese-origin consumables, such as electrosurgical electrodes and handpiece assemblies, have materially increased landed costs for distributors and healthcare providers. These measures, which impose duties as high as 25 percent on targeted categories, have compelled manufacturers to reassess their sourcing and pricing strategies to maintain competitiveness.⁵
However, in response to industry advocacy and regulatory review, the U.S. Trade Representative granted selective exemptions for a subset of medical devices, explicitly including vessel sealing and dividing instruments that utilize electrical energy for cutting and coagulation. These exclusions, which extend through mid-2026, have provided temporary relief for importers, helping to stabilize supply channels and avoid disruptions in critical surgical settings.⁶
Moreover, the legal challenge against emergency tariffs imposed under the International Emergency Economic Powers Act resulted in the U.S. Court of International Trade enjoining these duties, including the 25 percent surcharge on Canadian and Mexican goods. While this ruling has reinstated preferential access for North American-sourced medical devices, it also underscores the fluid nature of trade policy and the importance of dynamic risk management in global procurement.⁷
Uncovering Segmentation Insights on How Product Types Procedure Mix Channels Applications and End User Profiles Shape Vessel Sealing Device Adoption
In the vessel sealing arena, product-centric differentiation guides both hospital procurement and procedural preferences. Advanced bipolar systems have earned traction for their ability to deliver consistent energy delivery, particularly in handheld configurations favored for open and laparoscopic applications. Conversely, monopolar platforms-available as both reusable handpieces and single-use electrodes-offer cost flexibility for high-volume general and urologic surgeries, where procedural throughput is paramount. Ultrasonic devices, operating through endoscopic and handheld formats, continue to appeal in gynecologic and specialized cardiovascular interventions due to their precision and diminished thermal spread.
Procedural segmentation further illuminates device utilization patterns. Cardiovascular procedures consistently demand robust vessel sealing performance to manage high-pressure vessels, whereas general surgery relies on a balance of speed and reliability. In gynecologic and urologic settings, where anatomical variability and delicate tissue handling are critical, the choice of energy modality often dictates instrument selection and surgeon preference.
From a distribution standpoint, direct sales channels support deep clinical engagement, enabling manufacturers to align training and service with hospital systems’ requirements. Distributors expand geographic reach, ensuring availability in community hospitals and specialty centers, while online platforms cater to ambulatory surgical centers and emerging entrants by offering streamlined ordering and fulfillment.
Application-based insights reveal a dichotomy between laparoscopic procedures-dominated by general surgery and gynecologic cases-and open surgery interventions, where instrument robustness and cost-effectiveness drive purchasing decisions. End users span ambulatory surgical centers, where quick procedure turnaround is critical, to private and public hospitals and specialty clinics, each ecosystem shaping supplier relationships and device mix.
This comprehensive research report categorizes the Vessel Sealing Devices market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Product Type
- Procedure
- Application
- End User
- Sales Channel
Examining Regional Dynamics in the Americas Europe Middle East Africa and Asia Pacific That Influence Vessel Sealing Device Adoption and Growth
In the Americas, particularly within the United States, a mature healthcare infrastructure and a high volume of minimally invasive procedures underpin robust vessel sealing device adoption. The U.S. Food and Drug Administration’s streamlined approval pathways and consistent reimbursement frameworks have created a favorable environment for advanced energy platforms, resulting in widespread integration across hospital networks and ambulatory centers.⁸
The Europe, Middle East, and Africa region benefits from concerted investment in surgical innovation and regulatory harmonization under the European Medical Device Regulation. Initiatives such as the European Union’s Horizon Europe program have directed funding toward precision surgical technologies, including vessel sealing systems, encouraging manufacturers to localize development and collaborate with clinics to generate region-specific clinical evidence.⁹
Asia-Pacific is the fastest-growing market segment, propelled by government-driven healthcare expansions and rising medical expenditure. Favorable policies, combined with an increasing pool of trained surgeons and expanding hospital infrastructure across China, India, and Southeast Asia, have accelerated demand. The emphasis on minimally invasive techniques in tier-one hospitals and growing penetration in emerging economies underscore the region’s potential for long-term growth.¹⁰
This comprehensive research report examines key regions that drive the evolution of the Vessel Sealing Devices market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Insights into Leading Participants Showcasing Strategic Positioning Innovative Portfolios and Competitive Advantages in the Vessel Sealing Device Market
Major industry players have solidified their positions through portfolio diversification, service support frameworks, and strategic alliances. Medtronic remains a formidable presence, leveraging its LigaSure advanced bipolar instruments and generator platforms to address both open and minimally invasive procedures. The company’s ongoing focus on R&D and its expansive global distribution network sustain its leadership role.¹
Johnson & Johnson’s Ethicon division has bolstered its offering with the ENSEAL portfolio of curvilinear jaw tissue sealers and integrated generators, emphasizing ergonomics and seal quality in general and gynecologic surgery settings. Ethicon’s strategic acquisitions and regulatory clearances have reinforced its ability to deliver clinically robust solutions across varied procedural profiles.³
Olympus has introduced POWERSEAL bipolar devices and Thunderbeat systems, blending ultrasonic and bipolar energy to cater to complex laparoscopic interventions. The company’s emphasis on hybrid energy platforms and digital integration underscores its commitment to surgical precision and multidisciplinary applications.¹
Emerging innovators such as Bolder Surgical have differentiated with the CoolSeal vessel sealing platform, targeting niche endoscopic and handheld use cases with modular handpieces. Meanwhile, B. Braun and Erbe Elektromedizin offer generator-based solutions prized for reliability and serviceability in tertiary hospitals. Furthermore, Intuitive Surgical’s SynchroSeal, designed for robotic platforms, exemplifies the convergence of robotic and energy-based technologies, highlighting a burgeoning segment at the intersection of automation and vessel sealing.²
This comprehensive research report delivers an in-depth overview of the principal market players in the Vessel Sealing Devices market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Applied Medical Resources Corporation
- B. Braun Melsungen AG
- Baxter International Inc.
- Boston Scientific Corporation
- BOWA-electronic GmbH & Co. KG
- CONMED Corporation
- Cook Medical LLC
- Erbe Elektromedizin GmbH
- Hologic, Inc.
- Intuitive Surgical, Inc.
- Johnson & Johnson
- KARL STORZ SE & Co. KG
- KLS Martin Group
- Medtronic plc
- Olympus Corporation
- Richard Wolf GmbH
- Smith & Nephew plc
- Steris plc
- Stryker Corporation
- Terumo Corporation
Actionable Strategies and Roadmaps to Guide Industry Leaders toward Sustainable Growth Operational Excellence and Innovation in Vessel Sealing Technologies
Industry leaders should prioritize the development of hybrid energy platforms that seamlessly integrate bipolar and ultrasonic modalities, meeting the dual demands of speed and precision across diverse surgical settings. By expanding handpiece variations-reusable versus single-use-manufacturers can address both cost-efficiency and sterility concerns, tailoring solutions to institutional budgets and infection control protocols.
Strengthening digital connectivity through embedded sensors and cloud-based analytics can provide real-time device performance data, enabling predictive maintenance and usage optimization. By offering service contracts that incorporate remote monitoring, companies can deepen customer relationships and ensure uninterrupted clinical operations.
To mitigate trade policy risks and supply chain disruptions, organizations should diversify manufacturing footprints and establish dual-sourcing strategies for critical consumables. Engaging in proactive dialogue with regulatory bodies and industry associations can facilitate timely tariff exemptions and influence policy outcomes that affect medical device imports.
Finally, coupling clinical training programs with device launches-particularly in emerging markets and high-growth regions-will help solidify product adoption and generate real-world evidence. Collaborative partnerships with surgical societies and key opinion leaders can accelerate knowledge transfer, demonstrating the clinical advantages of advanced vessel sealing technologies.
Comprehensive Research Framework Integrating Primary Interviews Secondary Analysis and Rigorous Validation to Ensure Robust Vessel Sealing Device Market Insights
This report’s insights were derived through a multi-tiered research approach. Initially, secondary research encompassed an exhaustive review of regulatory filings, peer-reviewed literature, and industry publications to map the competitive landscape and identify prevailing trends. Market segment definitions and device classifications were validated against publicly available standards and registry databases.
Subsequently, primary research engaged a broad spectrum of stakeholders, including surgeons specializing in cardiovascular, general surgery, gynecology, and urology; procurement managers at hospitals and ambulatory surgical centers; and senior executives at key device manufacturers and distributors. These interviews provided nuanced perspectives on clinical preferences, purchasing considerations, and procedural outcomes.
Data triangulation and rigorous cross-verification were employed to reconcile conflicting insights and ensure the robustness of qualitative findings. Quantitative validation involved correlating device adoption trends with procedure volumes and hospital utilization rates, leveraging data from national health agencies and professional surgical associations.
Throughout the research process, segmentation logic was applied to differentiate product modalities, procedure types, sales channels, application settings, and end-user profiles, yielding a cohesive framework for understanding market dynamics and guiding strategic decision-making.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Vessel Sealing Devices market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Vessel Sealing Devices Market, by Product Type
- Vessel Sealing Devices Market, by Procedure
- Vessel Sealing Devices Market, by Application
- Vessel Sealing Devices Market, by End User
- Vessel Sealing Devices Market, by Sales Channel
- Vessel Sealing Devices Market, by Region
- Vessel Sealing Devices Market, by Group
- Vessel Sealing Devices Market, by Country
- United States Vessel Sealing Devices Market
- China Vessel Sealing Devices Market
- Competitive Landscape
- List of Figures [Total: 17]
- List of Tables [Total: 1749 ]
Concluding Reflections on Current Advancements Drivers and Emerging Opportunities Shaping the Future Trajectory of Vessel Sealing Device Adoption in Healthcare
The evolving vessel sealing device landscape underscores a trajectory toward increasingly sophisticated energy integration, digital connectivity, and surgical ergonomics. As the convergence of bipolar, ultrasonic, and robotic modalities continues, clinicians will benefit from enhanced precision, faster workflows, and improved patient safety.
Regional dynamics reinforce a dual narrative: established markets in North America and Europe set the benchmark for regulatory excellence and clinical evidence generation, while emerging markets in the Asia-Pacific region present high-growth opportunities, driven by healthcare infrastructure expansion and surgeon training initiatives.
Trade policy developments, from Section 301 tariff adjustments to temporary exemptions, highlight the critical need for agile supply chain management and proactive regulatory engagement. Companies that navigate these complexities effectively will secure preferential access to key markets and safeguard their competitive positions.
Ultimately, strategic imperatives for device manufacturers include harnessing hybrid energy platforms, deepening digital service offerings, and fostering robust clinical partnerships. By aligning innovation roadmaps with procedural demands and regional healthcare priorities, industry players can chart a course for sustained growth and leadership in the vessel sealing device domain.
Connect with Ketan Rohom to Unlock Tailored Vessel Sealing Device Market Insights and Elevate Your Strategic Decisions with Expert Guidance and Research Access
To explore this comprehensive vessel sealing device market research report and gain access to in-depth analysis, insights, and strategic guidance tailored to your organization’s needs, connect directly with Ketan Rohom, Associate Director, Sales & Marketing. By partnering with Ketan Rohom, you’ll secure a customized presentation of the report’s findings, discuss competitive landscape nuances, and identify the critical opportunities that will shape your next strategic decisions. Reach out to Ketan Rohom to arrange a detailed consultation and finalize your purchase of the full report today

- How big is the Vessel Sealing Devices Market?
- What is the Vessel Sealing Devices Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




