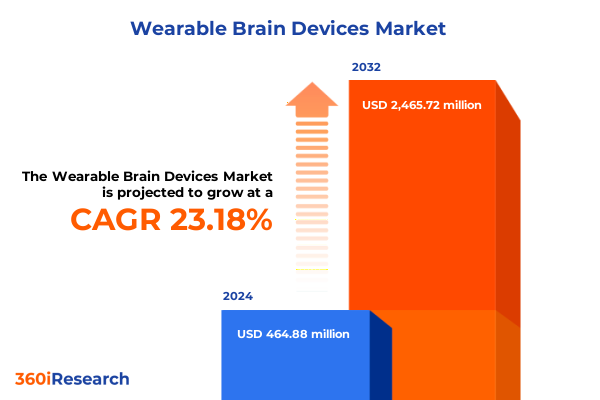
The Wearable Brain Devices Market size was estimated at USD 573.99 million in 2025 and expected to reach USD 694.01 million in 2026, at a CAGR of 23.14% to reach USD 2,465.72 million by 2032.
Emerging Frontiers in Wearable Brain Device Innovations Unlock New Pathways for Cognitive Enhancement Integration and Clinical Diagnostics Excellence
The wearable brain device market represents a convergence of neuroscience, engineering, and consumer electronics that is redefining how humans interact with technology. By seamlessly integrating neural interfaces into everyday life, these innovations promise to unlock new dimensions of human potential, offering unprecedented insights into cognitive function, mental well-being, and neural health.
Against a backdrop of aging populations, rising neurological disorder prevalence, and growing demand for personalized health solutions, wearable brain devices are emerging as transformative tools. They bridge the gap between diagnostic clinical applications and consumer wellness, creating a dynamic ecosystem where research, medical care, and lifestyle intersect to enhance quality of life and productivity.
Accelerated Disruption in Neurotechnology Propelled by Advances in AI Algorithms Miniaturization and Cross Sector Collaborations Igniting Market Evolution
The neurotechnology landscape is undergoing a seismic shift driven by breakthroughs in artificial intelligence, sensor miniaturization, and advanced materials science. Neural decoding algorithms powered by machine learning have evolved from laboratory curiosities into robust platforms capable of translating complex brain signals into actionable data, accelerating both medical and consumer applications in real time.
Concurrently, supply chain pressures stemming from geopolitical tensions and component scarcity are compelling manufacturers to rethink traditional sourcing models. The cost of critical raw components for wearable medical devices has surged by as much as twenty percent, prompting industry leaders to diversify suppliers and explore alternative manufacturing hubs to mitigate risk and maintain competitive pricing.
Moreover, strategic collaborations between tech giants, specialized neurotech startups, and academic institutions are fostering an ecosystem of open innovation. This collaborative framework is expediting regulatory approvals and establishing standardized protocols, ultimately reducing time to market and enhancing device safety and efficacy across invasive and noninvasive modalities.
Assessing the Comprehensive Economic and Supply Chain Consequences of US Section 301 Tariffs on Wearable Neural Interfaces and Associated Components in 2025
In January 2025, the United States Trade Representative enacted a new fifty percent tariff on semiconductor imports under Section 301, intensifying cost pressures on brain–computer interface components such as processors and signal amplifiers. This measure, aimed at bolstering domestic chip production, has inadvertently raised production costs across the noninvasive and implantable device segments, impacting manufacturers reliant on global supply chains.
Simultaneously, consumable medical device categories integral to invasive systems-including surgical masks, syringes, and gloves-have seen tariffs climb to rates between twenty-five and one hundred percent. Surgical and non-surgical facemasks now attract fifty percent duties in 2026, while syringes and needles face a one hundred percent levy since late 2024, creating downstream cost implications for hospitals, clinics, and research institutes deploying deep brain stimulators and implantable electrode arrays.
Faced with these headwinds, leading neurotechnology developers are evaluating nearshoring strategies and forging partnerships with domestic foundries to secure critical components. As supply chain fragmentation accelerates, organizations that proactively integrate supply diversification and vertical integration will be best positioned to preserve margins and ensure uninterrupted access to cutting-edge neural interface hardware.
Decoding Market Dynamics through Technology Application End User Distribution Channel and Price Range Segmentation to Unveil Strategic Growth Levers
A nuanced understanding of wearable brain device segmentation reveals five strategic dimensions shaping market development. Technology serves as a foundational axis, where invasive systems such as brain implants and deep brain stimulators coexist alongside noninvasive modalities including EEG, fNIRS, and MEG. Within noninvasive EEG, product variations further split into dry and wet sensor formats, each offering unique trade-offs in signal fidelity and user convenience.
Application-driven segmentation highlights distinct end-use ecosystems. Consumer-oriented solutions span gaming and wellness, with the latter encompassing fitness tracking, meditation, and sleep monitoring. In the medical realm, diagnostic devices address cognitive assessment and epilepsy detection, while rehabilitation and therapeutic applications target motor recovery, neurorehabilitation, depression treatment, and stroke rehabilitation. Research applications bifurcate into academic and corporate environments, reflecting diverse funding sources and innovation priorities.
End users range from individual consumers to healthcare providers and research institutes. Clinics and hospitals form the core of healthcare provider demand, with neurology clinics and rehabilitation centers driving specialized invasive device adoption. Distribution channels further diversify, including direct B2B and OEM sales, e-commerce platforms, and brick-and-mortar retail such as electronics and specialty stores. Finally, price range segmentation delineates economy, mid-range, and premium tiers, enabling targeted go-to-market strategies that align product features with willingness to pay.
This comprehensive research report categorizes the Wearable Brain Devices market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Technology
- Application
- End User
- Distribution Channel
Regional Market Variations Highlight Diverse Adoption Patterns and Regulatory Environments across Americas Europe Middle East Africa and Asia Pacific
Regional dynamics in the Americas underscore robust adoption of wearable brain technologies driven by strong research funding and a receptive consumer base. North America benefits from a mature healthcare infrastructure and supportive regulatory frameworks that facilitate rapid clinical trials and device approvals. Latin America, while nascent, is witnessing pilot programs in wellness and remote monitoring, laying the groundwork for broader deployment.
In Europe, Middle East & Africa, regulatory harmonization under EU directives has accelerated cross-border market entry, supporting a spectrum of neurotechnology trials from invasive BCI studies to noninvasive cognitive wellness devices. The EMEA region’s diverse socio-economic landscape, however, necessitates tailored pricing and distribution strategies to accommodate varying healthcare expenditure levels.
Asia-Pacific is emerging as both a manufacturing powerhouse and a burgeoning consumer market. China, Japan, and South Korea lead in advanced component production, while markets like India and Southeast Asia present significant growth potential for cost-effective, mid-range devices. Regional innovation hubs and government initiatives focused on AI and digital health are further catalyzing domestic R&D investments and commercial partnerships.
This comprehensive research report examines key regions that drive the evolution of the Wearable Brain Devices market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Competitive Landscape Insights Featuring Leading Invasive and Non Invasive Neural Interface Innovators Revolutionizing the Wearable Brain Device Market
The competitive landscape is defined by a dual track of invasive and noninvasive innovators, each pursuing distinct technological pathways. Invasive pioneers focus on direct neural modulation through implants, while noninvasive players emphasize accessible, user-friendly interfaces that cater to broad consumer and clinical markets. Strategic alliances and integration with software analytics platforms are becoming essential differentiators in this rapidly evolving environment.
In the invasive segment, Neuralink stands out with ambitious clinical and commercial milestones, targeting one billion dollars in annual revenue by 2031 and planning to implant neural chips in twenty thousand users per year across multiple clinical sites. Its FDA breakthrough designation for speech and vision restoration underscores regulatory progress, while Synchron’s minimally invasive Stentrode system, designed for implantation via the jugular vein, is expanding patient autonomy by enabling digital control through thought alone.
Among noninvasive solution providers, Emotiv has cemented its leadership with wireless EEG headsets and developer-friendly SDK ecosystems, evolving from saline-based wet sensors to advanced polymer-based dry electrodes for both research and consumer use. Kernel’s Flow platforms leverage time-domain fNIRS to deliver fMRI-like spatial resolution in a portable helmet, supporting clinical studies and psychedelic-medicine research with rapid setup times. Legacy medical device giants such as Medtronic and Boston Scientific remain influential in neurostimulation, collectively treating over a million patients worldwide and fostering innovation through extensive clinical networks.
This comprehensive research report delivers an in-depth overview of the principal market players in the Wearable Brain Devices market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Advanced Brain Monitoring Inc.
- ANT Neuro B.V.
- Blackrock Microsystems LLC
- Brain Products GmbH
- BrainCo Inc.
- BrainQ Technologies Ltd.
- Cadwell Industries Inc.
- Cognionics Inc.
- Compumedics Limited
- CorTec GmbH
- Emotiv Inc.
- InteraXon Inc.
- Kernel Inc.
- MindMaze SA
- MindMonitor Ltd.
- MyndPlay Ltd.
- Natus Medical Incorporated
- Neurable Inc.
- Neuroelectrics Barcelona SL
- NeuroSky Inc.
- NeuroWave Systems Inc.
- OpenBCI
- Ripple LLC
- Wearable Sensing Inc.
Actionable Strategies for Industry Stakeholders to Navigate Regulatory Complexities Enhance Collaboration and Secure Sustainable Growth in Neurotechnology
To navigate the intricate regulatory landscape, industry leaders should proactively engage with global standards bodies and contribute to the development of unified protocols for device safety, performance, and interoperability. Early alignment with regulatory agencies can expedite approval pathways and reduce time-to-market, particularly for invasive neural implants that require rigorous safety validation.
Supply chain resilience must be prioritized by diversifying component sourcing, negotiating strategic partnerships with domestic foundries, and investing in modular manufacturing capabilities. Organizations that establish flexible production networks will be better equipped to absorb tariff fluctuations and geopolitical disruptions while minimizing cost pass-through to end users.
Finally, fostering user-centric design through co-creation workshops and pilot programs can accelerate market adoption. By integrating patient and consumer feedback into product roadmaps, companies can optimize usability, enhance clinical outcomes, and differentiate offerings in a crowded marketplace. Strategic collaborations with software and AI analytics firms will further amplify the value proposition of neural data insights.
Rigorous Multi Source Research Methodology Integrating Primary Interviews Secondary Data and Quantitative Validation for Reliable Market Insights
Our research approach combined primary and secondary methodologies to ensure comprehensive market coverage and analytical rigor. Primary inputs included in-depth interviews with key opinion leaders, including neurologists, device developers, and regulatory experts, supplemented by a global survey of over a hundred decision-makers across healthcare providers, research institutions, and consumer electronics firms.
Secondary research involved systematic analysis of public filings, regulatory databases, peer-reviewed journals, and credible news outlets. Quantitative data was triangulated with proprietary industry data points to validate findings and refine segmentation models. Rigorous quality checks and peer reviews were conducted throughout the research process to guarantee accuracy and relevance.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Wearable Brain Devices market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Wearable Brain Devices Market, by Technology
- Wearable Brain Devices Market, by Application
- Wearable Brain Devices Market, by End User
- Wearable Brain Devices Market, by Distribution Channel
- Wearable Brain Devices Market, by Region
- Wearable Brain Devices Market, by Group
- Wearable Brain Devices Market, by Country
- United States Wearable Brain Devices Market
- China Wearable Brain Devices Market
- Competitive Landscape
- List of Figures [Total: 16]
- List of Tables [Total: 2544 ]
Synthesis of Critical Findings Emphasizing Technological Trajectories Supply Chain Resilience and Strategic Opportunities in Wearable Brain Device Markets
Wearable brain devices are at a pivotal juncture where technological innovation, regulatory evolution, and market dynamics converge to shape future growth trajectories. The interplay of invasive and noninvasive modalities, coupled with AI-driven signal processing, is unlocking new clinical and consumer applications, from therapeutic interventions to cognitive enhancement.
As tariff pressures and supply chain realignments introduce cost complexities, organizations that embrace strategic diversification, regulatory foresight, and user-driven design will capture the greatest share of emerging opportunities. The next wave of growth will be defined by seamless integration of neural interfaces into daily life, underpinned by robust ecosystems of data analytics and cross-sector collaboration.
Engaging with Associate Director of Sales and Marketing to Secure Comprehensive Wearable Brain Device Market Research Report and Drive Informed Decision Making
For a deeper dive into the strategic dynamics, technological breakthroughs, and market trajectories shaping the wearable brain device sector, reach out to Ketan Rohom, Associate Director, Sales & Marketing. He is available to guide you through the comprehensive research report designed to empower informed decisions, minimize risk, and optimize investment strategies. Engage now to secure your access to actionable insights that will drive your organization’s competitive advantage and accelerate growth in this rapidly evolving industry.

- How big is the Wearable Brain Devices Market?
- What is the Wearable Brain Devices Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




