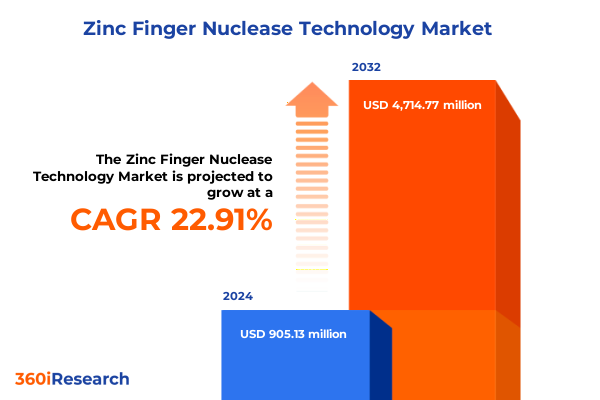
The Zinc Finger Nuclease Technology Market size was estimated at USD 1.10 billion in 2025 and expected to reach USD 1.35 billion in 2026, at a CAGR of 23.04% to reach USD 4.71 billion by 2032.
Zinc Finger Nucleases Unleashed: A Comprehensive Perspective on Genome Editing and Pioneering Applications in Biotechnology’s Frontier
Zinc Finger Nucleases represent one of the earliest and most versatile platforms for precision genome editing, enabling researchers to make targeted modifications within complex eukaryotic genomes. First pioneered by Sangamo BioSciences in the early 2000s, this technology harnesses engineered arrays of zinc finger domains fused to a nuclease domain to recognize and cleave specific DNA sequences, facilitating precise gene correction or disruption within living cells.
Over the past two decades, the zinc finger nuclease platform has been refined through advancements in protein engineering, linkers, and domain optimization. These improvements have yielded remarkable gains in specificity and efficiency, allowing applications that range from basic functional genomics research to clinical-stage therapies for genetic diseases. As regulatory agencies such as the FDA and EMA increasingly recognize gene editing as a cornerstone of personalized medicine, zinc finger nucleases are well positioned to complement newer modalities, offering unique advantages in compact size for viral vector delivery and modular design to target virtually any locus with high resolution.
Tracing the Revolutionary Trajectory of Zinc Finger Nuclease Technology Amid Paradigm-Altering Innovations and Regulatory Milestones
The evolutionary journey of zinc finger nuclease technology has been marked by transformative leaps in molecular design and clinical validation. A notable milestone occurred in 2019 when new architectures were described that reversed the conventional arrangement of DNA-binding and nuclease domains, combined with innovative linker chemistry to dramatically expand the diversity of targetable sequences by more than sixtyfold. This level of precision has enabled highly specific editing at multiple genomic loci, reducing off-target activity and opening doors to therapies for conditions such as sickle cell disease, hemophilia, and lysosomal storage disorders.
Simultaneously, the field has witnessed greater democratization of gene editing tools through open collaborations. In 2008, a consortium led by Keith Joung at Massachusetts General Hospital published a cost-effective, modular zinc finger nuclease platform that lowered barriers for academic and smaller biotech laboratories, thereby accelerating global research efforts. These collaborative shifts have not only driven innovation in the core platform but have also forged multidisciplinary partnerships that span agricultural biotechnology, industrial enzyme engineering, and next-generation therapeutic development.
Understanding the Ripple Effects of United States 2025 Tariffs on Zinc Finger Nuclease Supply Chains and Technology Adoption Dynamics
United States trade policies in 2025 have introduced a complex tariff landscape that significantly affects the supply chain for gene editing technologies, including zinc finger nucleases. A survey conducted by the Biotechnology Innovation Organization in March 2025 revealed that nearly 90 percent of U.S. biotech firms rely on imported components for at least half of their products, and proposed duties on clinical and laboratory imports are expected to drive up manufacturing costs and delay research timelines. In response to these pressures, companies are exploring alternative domestic raw material sources and redefining procurement strategies to maintain continuity in critical reagent supply.
Moreover, ongoing tensions with key trading partners have prompted Chinese and other international contract research organizations to recalibrate project planning and stockpile essential materials. For instance, WuXi AppTec and WuXi Biologics have publicly acknowledged adjustments to their global operations, including a shift toward local testing and a reevaluation of U.S. supply dependency to mitigate tariff-related risks. These cumulative impacts underscore the need for flexible sourcing, tariff pass-through clauses in supplier contracts, and accelerated efforts to re-onshore sensitive segments of the gene editing supply chain, ensuring robust access to enzyme platforms, plasmid backbones, and high-purity mRNA constructs.
Decoding Market Dynamics Through Multifaceted Segmentation of Product Types, Applications, End Users, and Therapeutic Focus Areas
A granular view of market dynamics emerges when dissecting product segmentation in the zinc finger nuclease ecosystem. The product typology spans mRNA based constructs, further differentiated into modified and unmodified mRNA formats for transient nuclease expression; plasmid based vectors available in both circular and linear configurations suitable for in vitro and ex vivo workflows; and protein based reagents encompassing both fusion proteins with tailored delivery domains and highly purified zinc finger nuclease proteins for direct application.
Layered atop product formats, applications shape demand across diverse sectors. Within agricultural biotechnology, gene editing is facilitating crop improvement and pest resistance traits, while in industrial biotechnology, biofuel production and enzyme engineering leverage targeted gene alterations for custom biocatalysts. Research usage remains a foundational pillar, with academic and contract research organizations driving protocol standardization. Meanwhile, therapeutic development bifurcates into ex vivo cell therapies and in vivo genome editing programs targeting monogenic and complex diseases.
End users of zinc finger nuclease solutions include academic and research institutes seeking bespoke editing platforms, contract research organizations integrating ZFNs into service offerings, and pharmaceutical and biotechnology companies deploying these tools for late-stage pipeline candidates. Therapeutic area segmentation further refines insight, encompassing genetic disorders-spanning both complex and single gene indications-infectious diseases that include bacterial and viral targets, and oncology indications ranging from hematological malignancies to solid tumor applications.
This comprehensive research report categorizes the Zinc Finger Nuclease Technology market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Product Type
- Application
- End User
- Therapeutic Area
Exploring Regional Nuances of Zinc Finger Nuclease Adoption Across the Americas, Europe Middle East Africa, and Asia Pacific Territories
Regional considerations play a pivotal role in the adoption and commercialization of zinc finger nuclease technology. In the Americas, the combination of robust private and public research funding alongside a streamlined FDA regulatory pathway has historically fostered early adoption, particularly in gene therapy clinical trials and ex vivo cell-based applications. This ecosystem benefits from established infrastructure for viral vector production and domestic reagent manufacturing, mitigating some tariff-induced sourcing disruptions as evidenced by domestic investment plans from leading manufacturers.
Europe, Middle East, and Africa present a varied regulatory mosaic shaped by the European Medicines Agency’s advanced therapy medicinal products guidelines and the EU’s unified ATMP framework, set to harmonize quality and clinical trial requirements effective July 2025. This convergence initiative aims to accelerate patient access to innovative therapies while maintaining rigorous safety standards-a crucial factor for in vivo genome editing programs and cross-border collaborative research.
Asia-Pacific is experiencing rapid growth, with China, Japan, and South Korea prioritizing gene editing innovation through government grants and CMC investments. However, heightened U.S. tariffs have spurred APAC-based players to diversify their reagent portfolios and strengthen local manufacturing to reduce dependence on imports, reflecting industry responses to supply chain stresses documented by both global survey data and corporate R&D adjustments.
This comprehensive research report examines key regions that drive the evolution of the Zinc Finger Nuclease Technology market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Profiling Leading Stakeholders Driving Zinc Finger Nuclease Innovations and Strategic Collaborations Across the Global Biotechnology Ecosystem
A review of key stakeholders underscores the strategic alliances and innovation pipelines shaping the zinc finger nuclease domain. Sangamo Therapeutics remains at the forefront, leveraging its proprietary zinc finger libraries and clinical candidates targeting hemophilia, MPS disorders, and sickle cell disease, with data demonstrating high on-target specificity and durable genome modifications. Collaborative agreements with pharmaceutical giants such as Genentech and partnerships in agricultural biotech projects further exemplify the technology’s cross-sector appeal and licensing potential.
Complementary to platform developers, life science tools companies including Thermo Fisher Scientific and Merck MilliporeSigma supply critical reagents, custom DNA-binding domains, and high-fidelity nucleases. Academic spinouts and bespoke gene editing service providers-such as those emerging from MGH’s Joung laboratory-offer competitive alternatives with open architectures, fostering price competition and broader accessibility for preclinical research. This dynamic supplier landscape challenges end users to balance cost, precision, and regulatory support when selecting a zinc finger nuclease solution.
This comprehensive research report delivers an in-depth overview of the principal market players in the Zinc Finger Nuclease Technology market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Cellectis SA
- Charles River Laboratories International, Inc.
- GenScript Biotech Corporation
- Integrated DNA Technologies, Inc.
- Lonza Group Ltd.
- Merck KGaA
- Revvity Discovery Limited
- Sangamo Therapeutics, Inc.
- Thermo Fisher Scientific Inc.
- ToolGen Inc.
- WuXi Biologics Co., Ltd.
Implementing Strategic Recommendations for Industry Leaders to Capitalize on Zinc Finger Nuclease Advances and Mitigate Emerging Operational Risks
Industry leaders seeking to harness the full potential of zinc finger nuclease technology must adopt a multi-pronged strategy. First, diversification of supply chains through proactive supplier qualification and local sourcing agreements can mitigate the cost volatility introduced by tariffs, while contractual tariff pass-through clauses preserve budget predictability. Second, fostering strategic partnerships-both academic and commercial-can accelerate platform enhancements, driving deeper specificity and expanded target range through co-development programs.
Further, engaging with regulatory bodies early in development to align on emerging guidelines for advanced therapy medicinal products ensures smoother clinical translation and market entry. The convergence of EMA and FDA expectations around CMC and clinical trial design presents a prime opportunity to harmonize dossiers for global filings and reduce time to approval. Finally, investment in next-generation chemistry, such as novel linker scaffolds and engineered nuclease domains, will be essential to stay ahead of CRISPR-based alternatives and maintain differentiation in high-value therapeutic niches.
Illuminating the Robust Research Methodology Underpinning Comprehensive Analysis of Zinc Finger Nuclease Market Insights and Trends
This executive summary is grounded in a rigorous research framework combining primary and secondary methodologies. Secondary data was systematically gathered from scientific literature, regulatory agency publications, patent filings, and reputable financial disclosures to map the evolution of zinc finger nuclease architectures and clinical pipelines. Key insights were triangulated through analysis of peer-reviewed journals, press releases, and industry news outlets to ensure currency and accuracy across global markets.
Primary research included in-depth interviews with thought leaders, senior R&D executives, and regulatory experts. These qualitative discussions provided nuanced perspectives on technology maturity, segment-specific challenges, and regional regulatory trajectories. Quantitative data points, where available, were obtained through proprietary survey instruments and corroborated against publicly accessible datasets to validate emerging trends. The synthesized findings were peer reviewed by external advisors to uphold methodological rigor and objectivity.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Zinc Finger Nuclease Technology market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Zinc Finger Nuclease Technology Market, by Product Type
- Zinc Finger Nuclease Technology Market, by Application
- Zinc Finger Nuclease Technology Market, by End User
- Zinc Finger Nuclease Technology Market, by Therapeutic Area
- Zinc Finger Nuclease Technology Market, by Region
- Zinc Finger Nuclease Technology Market, by Group
- Zinc Finger Nuclease Technology Market, by Country
- United States Zinc Finger Nuclease Technology Market
- China Zinc Finger Nuclease Technology Market
- Competitive Landscape
- List of Figures [Total: 16]
- List of Tables [Total: 2226 ]
Synthesizing Crucial Findings to Illuminate the Future Trajectory and Strategic Imperatives of Zinc Finger Nuclease Technology
Taken together, the insights presented in this summary highlight zinc finger nucleases as a proven, adaptable genome editing platform capable of addressing diverse research and therapeutic imperatives. Technological enhancements in specificity and delivery, combined with expanding regulatory frameworks for gene therapies, portend a robust future trajectory for ZFN-based interventions. While tariffs and supply chain disruptions pose near-term operational challenges, proactive risk mitigation and regional diversification strategies can preserve momentum and support sustained innovation.
Ultimately, stakeholders who integrate product segmentation insights, regional regulatory considerations, and strategic partnership models will be best positioned to capitalize on zinc finger nuclease technology. As gene editing continues to redefine the boundaries of precision medicine, zinc finger nucleases will remain a critical component of the gene editing toolbox-driving both scientific discovery and the next wave of groundbreaking therapies.
Unlock Exclusive Industry Insights on Zinc Finger Nuclease Market Trends and Engage with Ketan Rohom to Access the Complete Research Report Today
For immediate access to a comprehensive analysis of the Zinc Finger Nuclease market and to explore tailored insights that can empower strategic decision making, we invite you to reach out to Ketan Rohom, Associate Director of Sales and Marketing at 360iResearch. Engage directly with Ketan to discuss how this report can address your organization’s unique challenges, optimize your R&D investments, and position you at the forefront of gene editing innovation. Secure your copy today and harness the full potential of Zinc Finger Nuclease technology.

- How big is the Zinc Finger Nuclease Technology Market?
- What is the Zinc Finger Nuclease Technology Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




